(1S,4R)-4,7,7-Trimethyl-1-(1H-perimidin-2-yl)-2-oxabicyclo[2.2.1]heptan-3-one
Abstract
1. Introduction
2. Results and Discussion
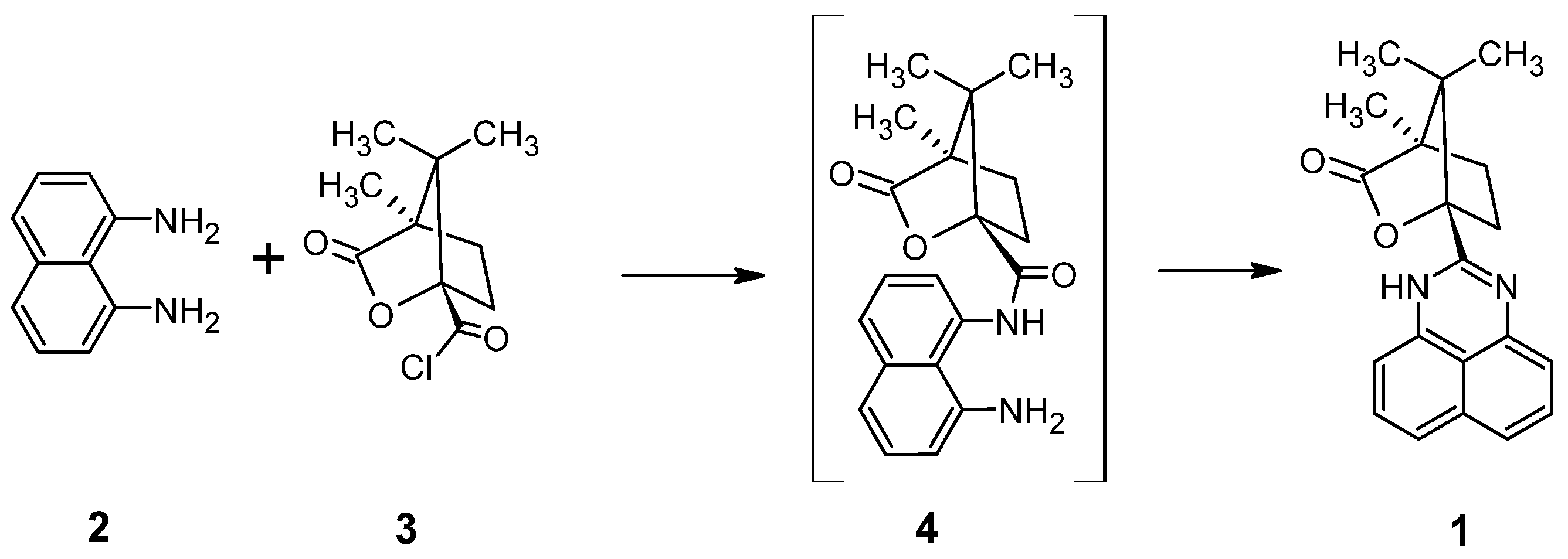
2.1. Synthesis
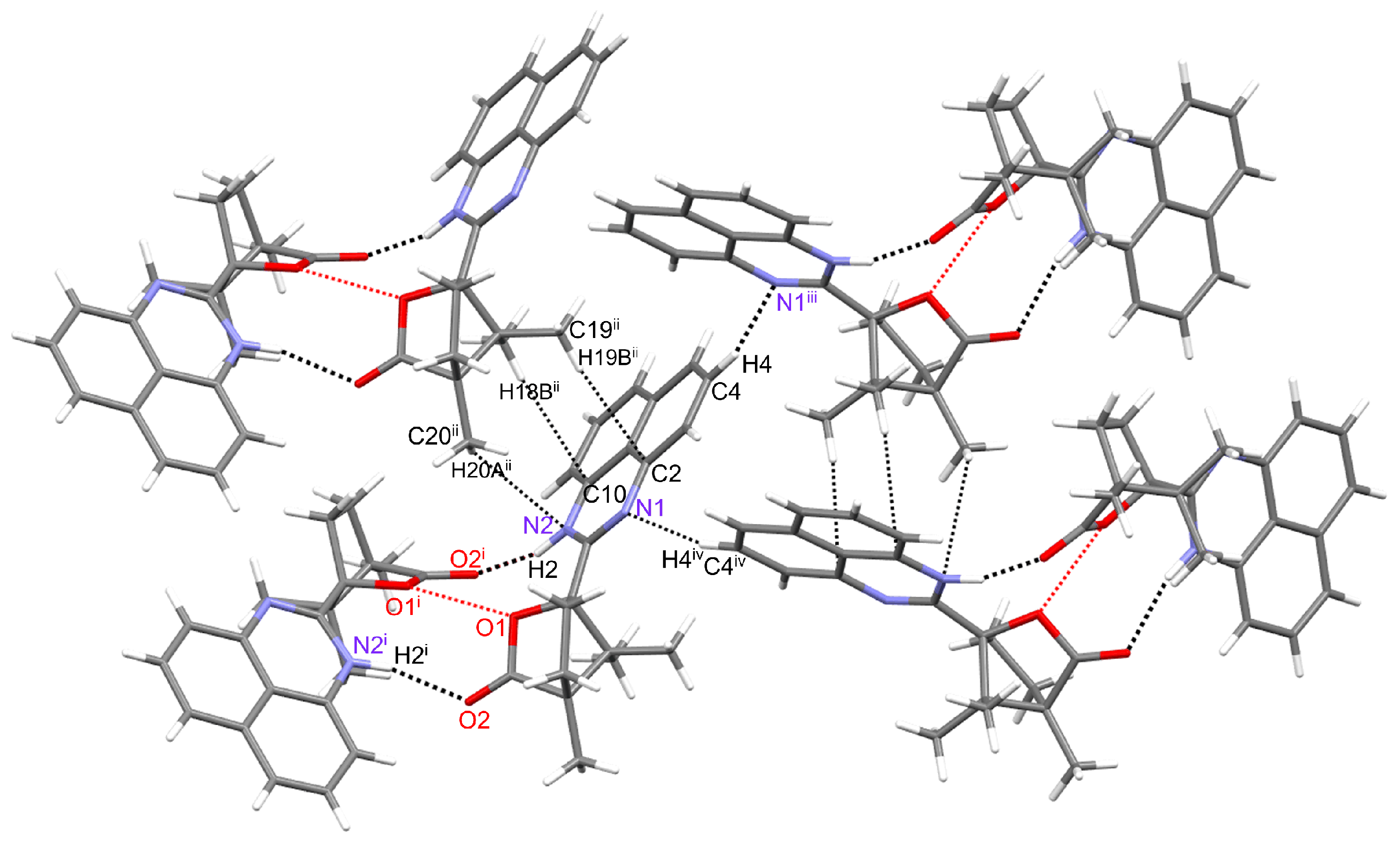
2.2. Molecular and Crystal Structure of 1
3. Materials and Methods
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalle, P.; Kiseleva, M.A.; Tatarin, S.V.; Smirnov, D.E.; Zakharov, A.Y.; Emets, V.V.; Churakov, A.V.; Bezzubov, S.I. A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-Perimidine. Molecules 2022, 27, 3201. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cai, J.; Mu, S.; Zhang, H.; Liu, K.; Liu, J.; Hong, J. Novel Perimidine Derivatives as Corrosion Inhibitors of HRB400 Steel in Simulated Concrete Pore Solution. Coatings 2023, 13, 73. [Google Scholar] [CrossRef]
- Czichy, M.; Janasik, P.; Wagner, P.; Officer, D.L.; Lapkowski, M. Electrochemical and Spectroelectrochemical Studies on the Reactivity of Perimidine–Carbazole–Thiophene Monomers towards the Formation of Multidimensional Macromolecules versus Stable π-Dimeric States. Materials 2021, 14, 2167. [Google Scholar] [CrossRef]
- Zhou, D.-C.; Lu, Y.-T.; Mai, Y.-W.; Zhang, C.; Xia, J.; Yao, P.-F.; Wang, H.-G.; Huang, S.-L.; Huang, Z.-S. Design, Synthesis and Biological Evaluation of Novel Perimidine o-Quinone Derivatives as Non-Intercalative Topoisomerase II Catalytic Inhibitors. Bioorg. Chem. 2019, 91, 103131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, S.; Roy, P.; Sondhi, S.M.; Sharma, A. Solvent-Free Synthesis and Anticancer Activity Evaluation of Benzimidazole and Perimidine Derivatives. Mol. Divers. 2018, 22, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Eldeab, H.A.; Eweas, A.F. A Greener Approach Synthesis and Docking Studies of Perimidine Derivatives as Potential Anticancer Agents. J. Heterocycl. Chem. 2018, 55, 431–439. [Google Scholar] [CrossRef]
- Goge, M.N.; Sithebe, S.; Papo, T.R. 2-[2-(Diphenylphosphoryl)Phenyl]-1H-Perimidine. Molbank 2022, 2023, M1537. [Google Scholar] [CrossRef]
- Petkova, Z.; Rusew, R.; Bakalova, S.; Shivachev, B.; Kurteva, V. 2-(1H-Imidazol-2-Yl)-2,3-Dihydro-1H-Perimidine. Molbank 2023, 2023, M1587. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta. Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
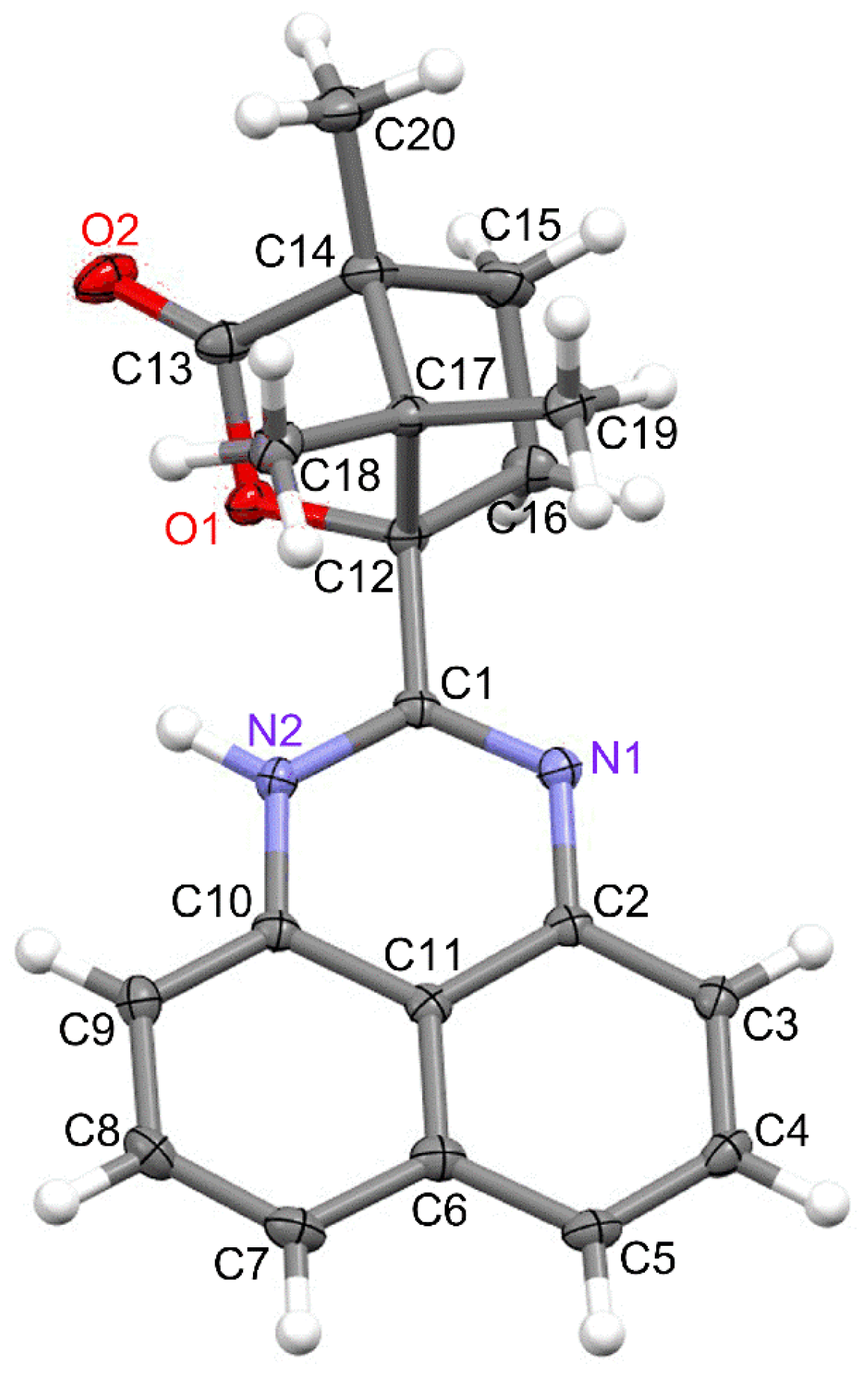
| Bond Lengths | Angles | ||
|---|---|---|---|
| C12—O1 | 1.475(2) | O1—C13—O2 | 122.0(2) |
| C13—O1 | 1.359(2) | C12—O1—C13 | 106.0(1) |
| C13—O2 | 1.206(2) | O1—C12—C17 | 101.6(1) |
| C1—N1 | 1.296(2) | O2—C13—C14 | 130.0(2) |
| C2—N1 | 1.411(2) | C12—C17—C14 | 91.5(1) |
| C1—N2 | 1.364(2) | C13—C14—C17 | 98.7(1) |
| C10—N2 | 1.399(2) | C18—C17—C19 | 109.1(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speina, E.; Łyczko, K.; Mieczkowski, A. (1S,4R)-4,7,7-Trimethyl-1-(1H-perimidin-2-yl)-2-oxabicyclo[2.2.1]heptan-3-one. Molbank 2025, 2025, M2111. https://doi.org/10.3390/M2111
Speina E, Łyczko K, Mieczkowski A. (1S,4R)-4,7,7-Trimethyl-1-(1H-perimidin-2-yl)-2-oxabicyclo[2.2.1]heptan-3-one. Molbank. 2025; 2025(4):M2111. https://doi.org/10.3390/M2111
Chicago/Turabian StyleSpeina, Elżbieta, Krzysztof Łyczko, and Adam Mieczkowski. 2025. "(1S,4R)-4,7,7-Trimethyl-1-(1H-perimidin-2-yl)-2-oxabicyclo[2.2.1]heptan-3-one" Molbank 2025, no. 4: M2111. https://doi.org/10.3390/M2111
APA StyleSpeina, E., Łyczko, K., & Mieczkowski, A. (2025). (1S,4R)-4,7,7-Trimethyl-1-(1H-perimidin-2-yl)-2-oxabicyclo[2.2.1]heptan-3-one. Molbank, 2025(4), M2111. https://doi.org/10.3390/M2111






