Synthesis and Characterization of Three New Furan-Containing Terpyridine Derivatives
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
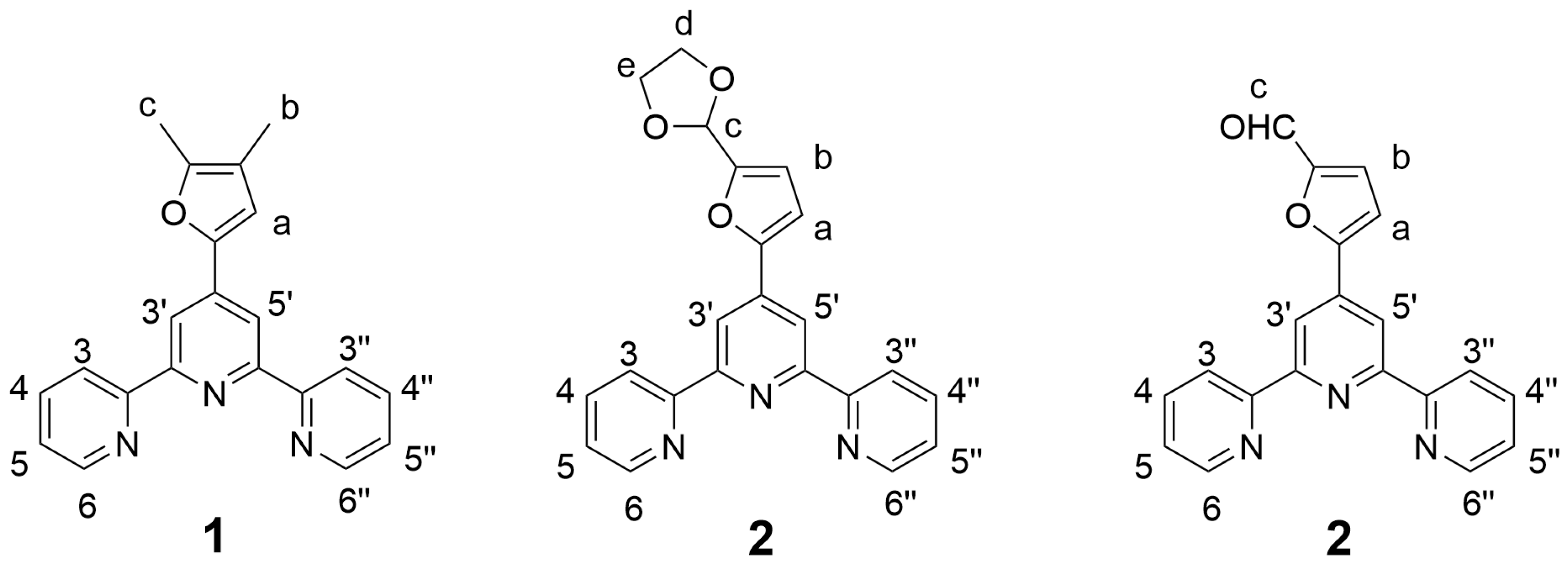
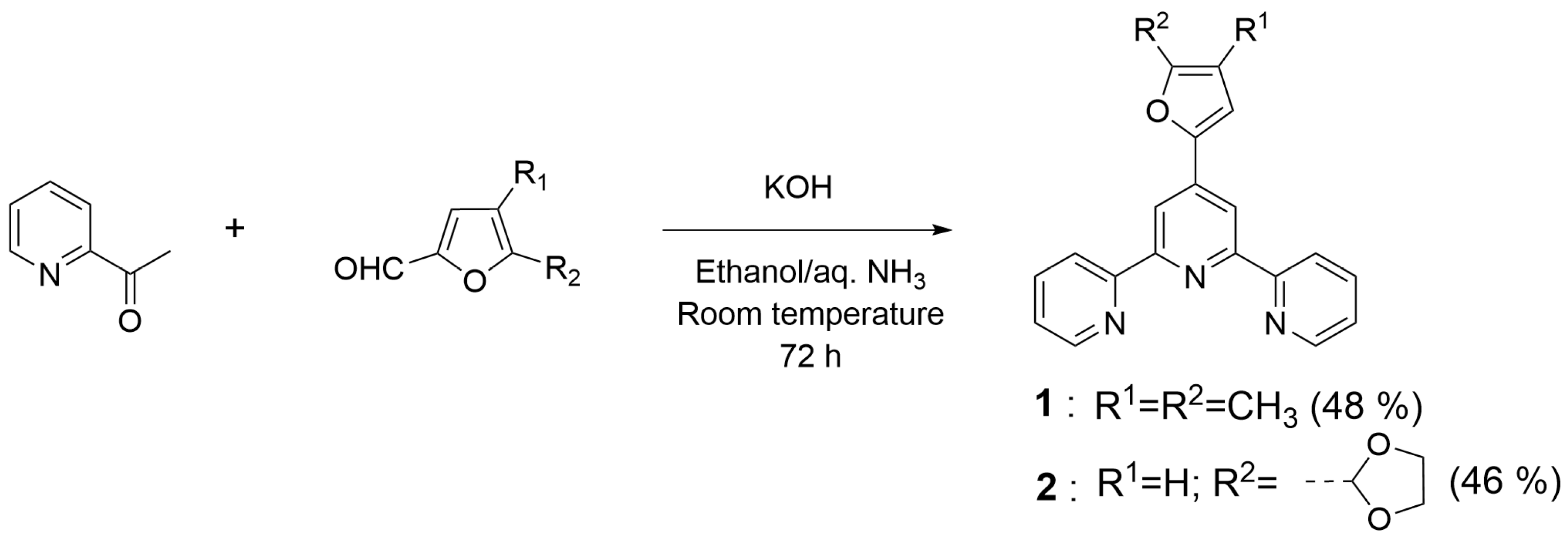
- 4′-(4,5-Dimethylfuran-2-yl)-2,2′:6′,2″-terpyridine (1): 4,5-dimethylfuran-2-furaldehyde (5.00 g; 40.3 mmol), 85% potassium hydroxide pellets (6.21 g; 94.1 mmol), and 25% aqueous ammonia solution (120 mL) were successively added to a solution of 2-acetylpyridine (9.76 g; 80.6 mmol) in absolute ethanol (200 mL). The reaction mixture was stirred at room temperature for 72 h. The precipitated solid was collected by filtration and washed with ice-cold 50% ethanol until washings were colorless. The crude product was air-dried and 1 is obtained as a yellow solid (6.34 g; 48%) that was analytically pure by 1H NMR. m.p. = 222.1–223.1 °C. 1H-NMR (CDCl3, 400 MHz), δ (ppm): 8.72 (d, 2H, H6, 6″, J = 4.8 Hz), 8.62 (m, 4H, H3, 3″, 3′, 5′), 7.84 (td, 2H, H4, 4″, J = 7.6 Hz, J = 1.8 Hz), 7.32 (ddd, 2H, H5, 5″, J = 7.4 Hz, J = 4.8 Hz, J = 1.1 Hz), 6.90 (s, 1H, Ha), 2.32 (s, 3H, Hb), 2.00 (s, 3H, Hc). 13C-NMR (CDCl3, 100 MHz), δ (ppm): 156.3, 155.7, 149.7, 149.1, 148.9, 139.8, 136.8, 123.7, 121.3, 116.9, 114.5, 112.8, 11.7, 9.9. HR-MS: calc. for [C21H17N5O + H]+ 328.14444, found 328.14412. IR (ATR): νmax (cm−1): 3052.5, 1583.9, 1396.0, 791.3.
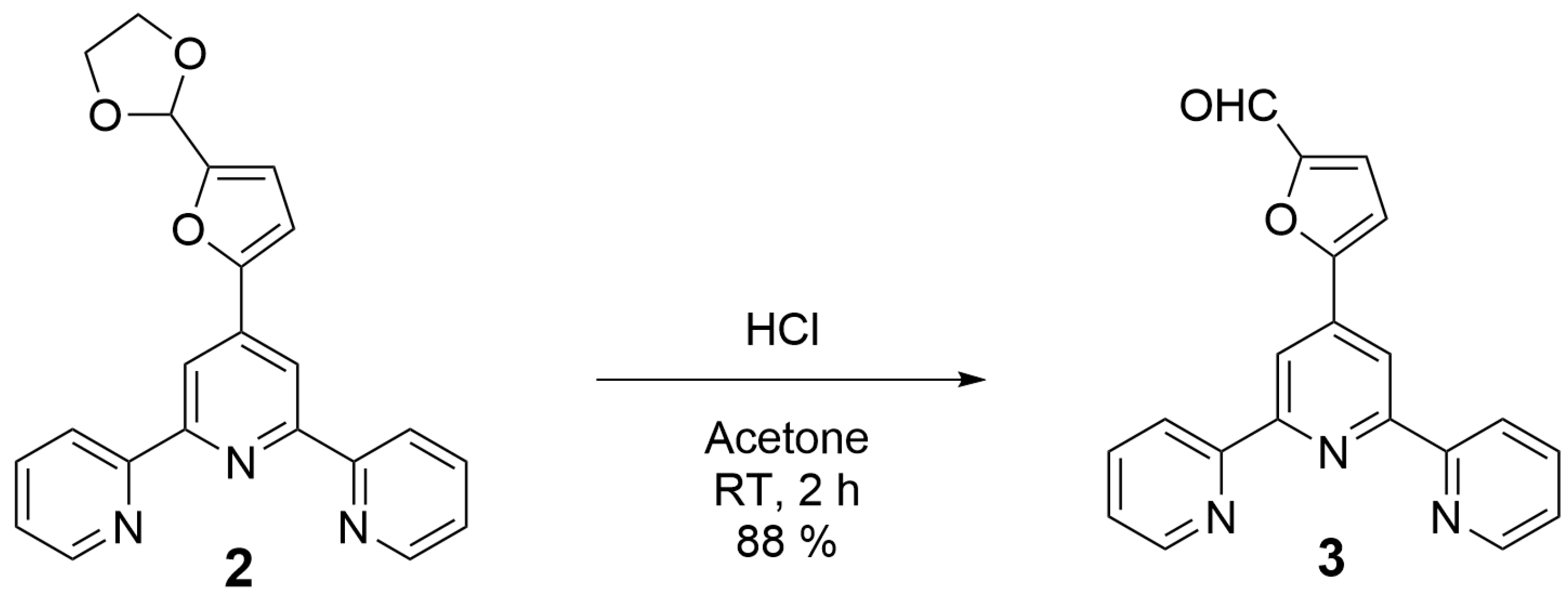
- 4′-((1,3-Dioxolan-2-yl)furan-2-yl)-2,2′:6′,2″-terpyridine (2): 5-(1,3-Dioxolan-2-yl)-2-furaldehyde (1.92 g; 11.4 mmol), 85% potassium hydroxide pellets (1.76 g; 26.7 mmol), and 25% aqueous ammonia solution (34 mL) were successively added to a solution of 2-acetylpyridine (2.76 g; 22.8 mmol) in absolute ethanol (60 mL). The reaction mixture was stirred at room temperature for 72 h. The precipitated solid was collected by filtration and washed with ice-cold 50% ethanol until washings were colorless. The crude product was air-dried and 2 was obtained as a white solid (1.96 g; 46%) that was analytically pure by 1H NMR. m.p. = 114.4–115.4 °C. 1H-NMR (CDCl3, 400 MHz), δ (ppm): 8.72 (dd, 2H, H6, 6″, J = 4.8 Hz, J = 0.8 Hz), 8.70 (s, 2H, H3′, 5′), 8.62 (d, 2H, H3, 3″, J = 8.0 Hz), 7.85 (td, 2H, H4, 4″, J = 7.7 Hz, J = 1.8 Hz), 7.34 (ddd, 2H, H5, 5″, J = 7.4 Hz, J = 4.8 Hz, J = 1.1 Hz), 7.07 (d, 1H, Hb, J = 3.4 Hz), 6.60 (d, 1H, Ha, J = 3.4 Hz), 6.06 (s, 1H, Hc), 4.18 (m, 2H, Hd), 4.06 (m, 2H, He). 13C-NMR (CDCl3, 100 MHz), δ (ppm): 156.0, 155.8, 152.7, 152.4, 149.0, 139.3, 137.0, 124.0, 121.4, 115.3, 110.6, 109.7, 97.8, 65.2. HR-MS: calc. for [C22H17N3O3 + H]+ 372.13427, found 372.13380. IR (ATR): νmax (cm−1): 2892.3, 1585.7, 1410.0, 1113.5, 785.2.
- 4′-(5-Formylfuran-2-yl)-2,2′:6′,2″-terpyridine (3): To a solution of 2 (1.00 g; 2.7 mmol) in acetone is added conc. hydrochloric acid (3 mL). The obtained suspension is stirred at room temperature for 2 h. The solvent is removed under vacuo and the residue is taken up in water (100 mL). The pH of the solution is adjusted to 9 by the portion-wise addition of solid sodium carbonate. The resulting precipitate is filtered, washed with water and air-dried. Analytically pure 3 is obtained as a white solid (0.78 g; 88%). m.p. = 227.6–229.1 °C. 1H-NMR (CDCl3, 400 MHz), δ (ppm): 9.78 (s, 1H, Hc), 8.82 (s, 2H, H3′, 5′), 8.73 (dd, 2H, H6, 6″, J = 4.7 Hz, J = 0.7 Hz), 8.62 (d, 2H, H3, 3″, J = 8.0 Hz), 7.87 (td, 2H, H4, 4″, J = 7.7 Hz, J = 1.8 Hz), 7.36 (m, 3H, H5, 5″, Hb), 7.25 (d, 1H, Ha, J = 1.4 Hz). 13C-NMR (CDCl3, 100 MHz), δ (ppm): 178.1, 156.8, 156.3, 155.5, 153.0, 149.1, 138.0, 137.1, 124.2, 121.9, 121.4, 116.3, 111.2. HR-MS: calc. for [C20H13N3O2 + H]+ 328.10805, found 328.10775. IR (ATR): νmax (cm−1): 3063.3, 3021.5, 2829.1, 1671.0, 1587.2, 1513.4, 1404.8, 786.9.
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Sciences Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef]
- Han, N.; Jiang, X.; Wang, M. Novel metallo-supramolecular architectures based on side-pyridine-modified terpyridines: Design, self-assembly, and properties. Chem. Sci. 2025, 16, 19532–19569. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Ebralidze, I.I.; Zenkina, O.V. Polypyridine-based architectures for smart electrochromic and energy storage materials. Can. J. Chem. 2023, 101, 400–417. [Google Scholar] [CrossRef]
- Kainat, S.F.; Hawsawi, M.B.; Mughal, E.U.; Naeem, N.; Almohyawi, A.M.; Altass, H.M.; Hussein, E.M.; Sadiq, A.; Moussa, Z.; Abd-El-Aziz, A.S. Recent developments in the synthesis and applications of terpyridine-based metal complexex: A systematic review. RSC Adv. 2024, 14, 21464–21537. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Knorr, M. Syntheses and applications of furanyl-functionalised 2,2′:6′,2″-terpyridines. Beilstein J. Org. Chem. 2012, 8, 379–389. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4″-tricarboxy-2,2′:6′,2″-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Constable, E.C.; Dunphy, E.L.; Housecroft, C.E.; Neuburger, M.; Schaffner, S.; Schaper, F.; Batten, S.R. Expanded ligands: Bis(2,2′:6′,2″-terpyridine carboxylic acid)ruthenium(II) complexes as metallosupramolecular analogues of dicarboxylic acids. Dalton Trans. 2007, 38, 4323–4332. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Lin, H.; Wang, R.; Zhang, L.; Sun, D. Synthesis, structure, and properties of a 3D porous Zn(II) MOF constructed from a terpyridine-based ligand. RSC Adv. 2016, 6, 16575–16580. [Google Scholar] [CrossRef]
- Etienne, S.; Beley, M. Preparation and study of ruthenium(II) 2,2′:6′,2″-terpyridine complexes linked by an O-CH2 spacer to thiophene moieties. Inorg. Chem. Commun. 2006, 9, 68–71. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Jiang, J.; Huang, J.; Chen, H.; Pan, L.; Nesterov, D.S.; Ma, Z.; Pombeiro, A.J.L. A New Concept of Enhancing the Anticancer Activity of Manganese Terpyridine Complex by Oxygen-Containing Substituent Modification. Int. J. Mol. Sci. 2023, 24, 3903. [Google Scholar] [CrossRef]
- Czerwinska, K.; Machura, B.; Kula, S.; Krompiec, S.; Erfurt, K.; Roma-Rodrigues, C.; Fernandes, A.R.; Shul’pina, L.; Ikonnikov, N.S.; Shul’pin, G.B. Copper(II) complexes of functionalized 2,2′:6′,2″-terpyridines and 2,6-di(thiazol-2-yl)pyridine: Structure, spectroscopy, cytotoxicity and catalytic activity. Dalton Trans. 2017, 46, 9591–9604. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.-W.; Peng, D.; Li, Y.-Q.; Shen, J.-R.; Li, S.-H. Syntheses, crystal structures and DNA-binding activities of divalent Fe, Cu, Zn and Cd complexes with 4′-(furan-2-yl)-2,2′:6′,2″-terpyridine. Z. Für Naturforschung B. 2017, 72, 687–695. [Google Scholar] [CrossRef]
- Kröhnke, F. Synthesis using pyridinium salts.5.Specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Francisco, T.N.; Albuquerque, H.M.T.; Silva, A.M.S. An In-Depth Exploration of Six Decades of the Kröhnke Pyridine Synthesis. Chem. Eur. J. 2024, 30, e202401672. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2″-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Jouaiti, A. Terpyridinebenzaldehyde isomers: One-pot facile synthesis. Synth. Commun. 2021, 51, 1547–1555. [Google Scholar] [CrossRef]
- Beley, M.; Delabouglise, D.; Houppy, G.; Husson, J.; Petit, J.-P. Preparation and properties of ruthenium (II) complexes of 2,2′:6′,2″-terpyridines substituted at the 4′-position with heterocyclic groups. Inorg. Chim. Acta 2005, 358, 3075–3083. [Google Scholar] [CrossRef]
- Sonia; Khatua, S.; Samanta, S.; Mongal, B.N. Synthesis, Characterization, Optical, Electrochemical, and Theoretical Studies of Substituted Terpyridines. Polycycl. Aromat. Comp. 2023, 44, 3408–3418. [Google Scholar] [CrossRef]
- Husson, J.; Migianu, E.; Beley, M.; Kirsch, G. Synthesis of New Terpyridines under Solventless Conditions Using Alumina. Synthesis 2004, 2, 267–270. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank 2018, 2018, M1032. [Google Scholar] [CrossRef]
- Conradie, J.; Erasmus, E. Cobalt complexes with multi-dentate N-donor ligands: Redox, X-ray photoelectron spectroscopic and theoretical study. Results Chem. 2023, 5, 100818. [Google Scholar] [CrossRef]
- Cook, G.L.; Church, F.M. Correlations of the infrared spectra of some pyridines. J. Phys. Chem. 1957, 61, 458–462. [Google Scholar] [CrossRef]
- Constable, E.C.; Redondo, A.H.; Housecroft, C.E.; Neuburger, M. Aldehyde-decorated 2,2′-bipyridine and 2,2′:6′,2″-terpyridine ruthenium(II) complexes: Convenient scaffolds for supramolecular chemistry. Inorg. Chem. Commun. 2010, 13, 70–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J. Synthesis and Characterization of Three New Furan-Containing Terpyridine Derivatives. Molbank 2025, 2025, M2098. https://doi.org/10.3390/M2098
Husson J. Synthesis and Characterization of Three New Furan-Containing Terpyridine Derivatives. Molbank. 2025; 2025(4):M2098. https://doi.org/10.3390/M2098
Chicago/Turabian StyleHusson, Jérôme. 2025. "Synthesis and Characterization of Three New Furan-Containing Terpyridine Derivatives" Molbank 2025, no. 4: M2098. https://doi.org/10.3390/M2098
APA StyleHusson, J. (2025). Synthesis and Characterization of Three New Furan-Containing Terpyridine Derivatives. Molbank, 2025(4), M2098. https://doi.org/10.3390/M2098






