[1,2,5]Thiadiazolo[3,4-b]pyrazine-5,6(4H,7H)-dione
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harris, S.L.; Dutta, S.; Liu, N.; Wollenberg, T.; Wang, X. Extended structure-activity relationship studies of the [1,2,5]oxadiazolo[3,4-b]pyrazine-containing colistin adjuvants. Bioorg. Med. Chem. Lett. 2025, 115, 130008. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J.E.; Salamoun, J.M.; Garcia, C.J.; Hargett, S.; Beretta, M.; Shrestha, R.; Li, C.; Hoehn, K.L.; Santos, W.L. Unsymmetric hydroxylamine and hydrazine bam15 derivatives as potent mitochondrial uncouplers. Bioorg. Med. Chem. 2025, 118, 118045. [Google Scholar] [CrossRef] [PubMed]
- Billen, M.; Reynders, S.; Claes, S.; Kleinboelting, S.; Rozenski, J.; Bulai, R.-G.; Rocca, E.; Homer, N.Z.M.; Webster, S.P.; Kaminski, T.P.; et al. Discovery and exploration of disubstituted [1,2,5]oxadiazolo-[3,4-b]pyrazines as novel c-c chemokine receptor type 5 signaling inhibitors targeting the intracellular allosteric binding pocket. Eur. J. Med. Chem. 2025, 291, 117600. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Gomez-Gutierrez, P.; Campos, P.M.; Vega, M.; Messeguer, A.; Perez, J.J. Structure-activity studies of novel di-substituted [1,2,5]oxadiazolo [3,4-b]pyrazine analogs targeting the a-loop regulatory site of p38 map kinase. Curr. Med. Chem. 2022, 29, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Tang, J.; Zhang, Q.; Cheng, G.; Yang, H. Toward high-energy and low-sensitivity energetic materials based on a fused [5,5,5,6]-tetracyclic backbone. Org. Lett. 2023, 25, 3487–3491. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.C. 1,2,5-Thiadiazolo[3,4-b]pyrazines. J. Heterocycl. Chem. 1975, 12, 451–453. [Google Scholar] [CrossRef]
- Zhiqiang, F.; Zhufang, S.; Yi, H.; Xupeng, H.; Xing, W.; Chao, Y.; Caina, L. Pyrazine Derivative and Preparation Method and Pharmaceutical Composition and Purpose Thereof. Patent CN108929280, 4 December 2018. [Google Scholar]
- Yamashita, Y.; Saito, K.; Suzuki, T.; Kabuto, C.; Mukai, T.; Miyashi, T. Bis([1,2,5]thiadiazolo)[3,4-b ;3′,4′-e]pyrazin, ein neuer heterocyclus mit 14 π-elektronen und hoher elektronenaffinität. Angew. Chem. 1988, 100, 428–429. [Google Scholar] [CrossRef]
- Dudey, A.P.; Rigby, J.M.; Hughes, G.R.; Stephenson, G.R.; Storr, T.E.; Chantry, A.; Hemmings, A.M. Expanding the inhibitor space of the wwp1 and wwp2 hect e3 ligases. J. Enzyme Inhib. Med. Chem. 2024, 39, 2394895. [Google Scholar] [CrossRef] [PubMed]
- Starchenkov, I.B.; Andrianov, V.G. Chemistry of furazano[3,4-b]pyrazines. Chem. Heterocycl. Compd. 1997, 33, 1219–1233. [Google Scholar] [CrossRef]
- Hoehn, K.; Santos, W.L.; Childress, E.S.; Dai, Y.; Murray, J.; Santiago-Rivera, J. Compositions and Methods for Preparing and Using Mitochondrial Uncouplers. Patent WO2018/217757, 22 May 2018. [Google Scholar]
- Camilleri, P.; Odell, B.; O’Neill, P. Electrochemical properties of pyrazinothiadiazoles. J. Chem. Soc. Perkin Trans. 2 1987, 1671. [Google Scholar] [CrossRef]
- Childress, E.S.; Salamoun, J.M.; Hargett, S.R.; Alexopoulos, S.J.; Chen, S.-Y.; Shah, D.P.; Santiago-Rivera, J.; Garcia, C.J.; Dai, Y.; Tucker, S.P.; et al. [1,2,5]Oxadiazolo[3,4-b]pyrazine-5,6-diamine derivatives as mitochondrial uncouplers for the potential treatment of nonalcoholic steatohepatitis. J. Med. Chem. 2020, 63, 2511–2526. [Google Scholar] [CrossRef] [PubMed]
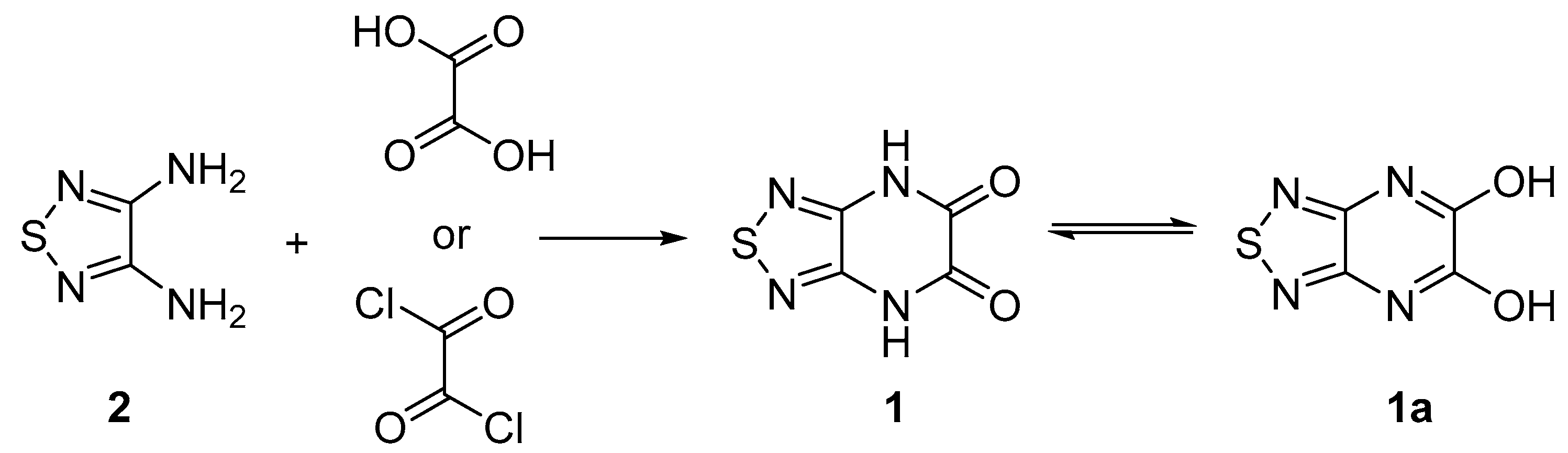
| Entry | Reagent | Solvent | Time, h | Yield of 1, % |
|---|---|---|---|---|
| 1 | oxalic acid | dil. HCl | 3 | 12 |
| 2 | oxalic acid | dil. HCl | 6 | 51 |
| 3 | oxalyl chloride | glacial AcOH | 5 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, L.S.; Obruchnikova, N.V.; Rakitin, O.A. [1,2,5]Thiadiazolo[3,4-b]pyrazine-5,6(4H,7H)-dione. Molbank 2025, 2025, M2030. https://doi.org/10.3390/M2030
Konstantinova LS, Obruchnikova NV, Rakitin OA. [1,2,5]Thiadiazolo[3,4-b]pyrazine-5,6(4H,7H)-dione. Molbank. 2025; 2025(3):M2030. https://doi.org/10.3390/M2030
Chicago/Turabian StyleKonstantinova, Lidia S., Natalia V. Obruchnikova, and Oleg A. Rakitin. 2025. "[1,2,5]Thiadiazolo[3,4-b]pyrazine-5,6(4H,7H)-dione" Molbank 2025, no. 3: M2030. https://doi.org/10.3390/M2030
APA StyleKonstantinova, L. S., Obruchnikova, N. V., & Rakitin, O. A. (2025). [1,2,5]Thiadiazolo[3,4-b]pyrazine-5,6(4H,7H)-dione. Molbank, 2025(3), M2030. https://doi.org/10.3390/M2030







