Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate
Abstract
1. Introduction
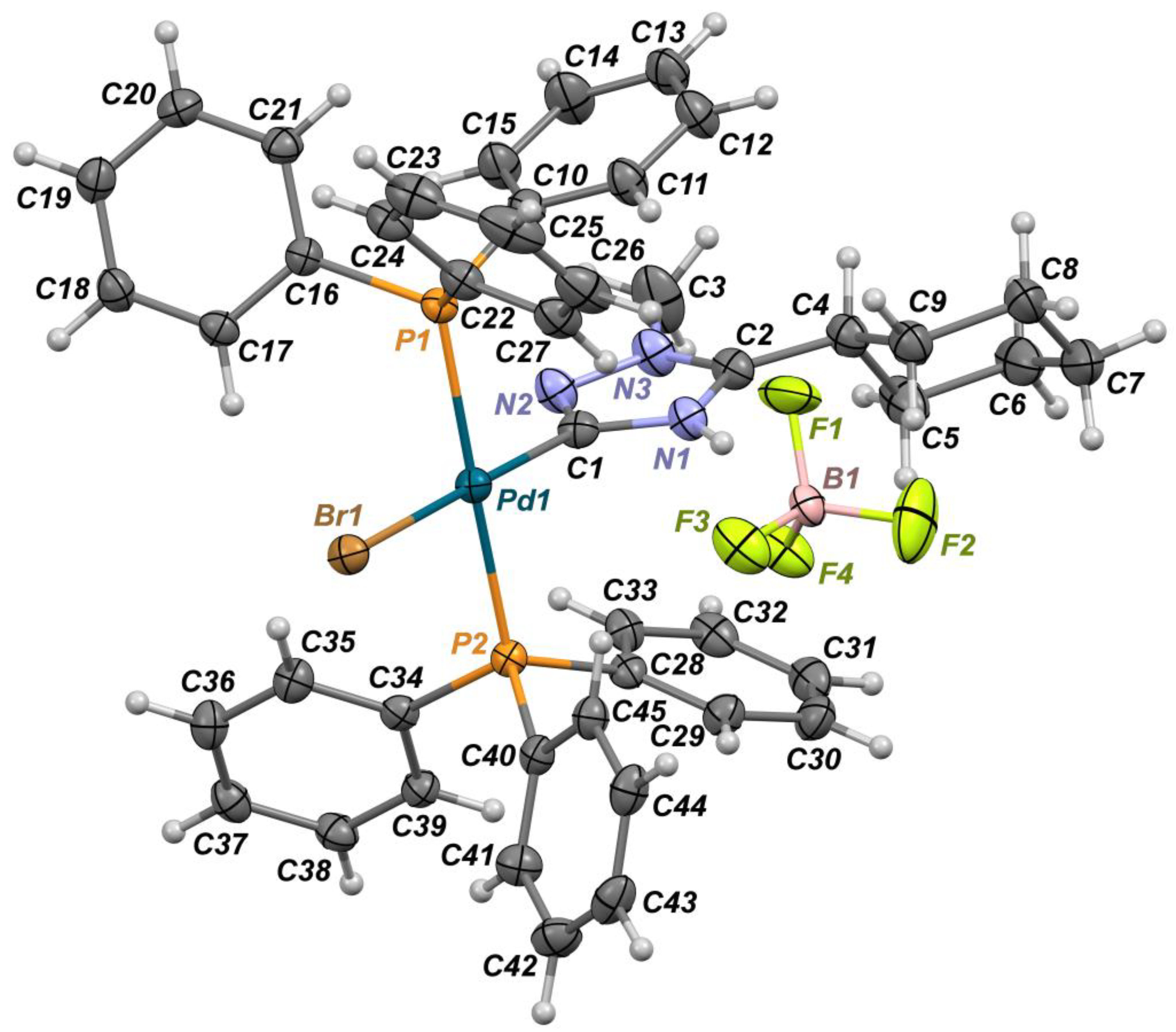
2. Results and Discussion
Synthesis
3. Materials and Methods
3.1. Synthesis of 3-Bromo-5-cyclohexyl-1-methyl-1H-1,2,4-triazole (2)
3.2. Synthesis of Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate (3)
3.3. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahnke, M.C.; Hahn, F.E. Complexes Bearing Protic N-Heterocyclic Carbenes: Synthesis and Applications. Chem. Lett. 2015, 44, 226–237. [Google Scholar] [CrossRef]
- Kuwata, S.; Hahn, F.E. Complexes Bearing Protic N-Heterocyclic Carbene Ligands. Chem. Rev. 2018, 118, 9642–9677. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.Y.; Ho, C.C.; Gardiner, M.G.; Bissember, A.C. Non-Classical Anionic Naked N-Heterocyclic Carbenes: Fundamental Properties and Emerging Applications in Synthesis and Catalysis. Catalysts 2018, 8, 620. [Google Scholar] [CrossRef]
- Zhu, J.; Lindsay, V.N.G. Benzimidazolyl Palladium Complexes as Highly Active and General Bifunctional Catalysts in Sustainable Cross-Coupling Reactions. ACS Catal. 2019, 9, 6993–6998. [Google Scholar] [CrossRef]
- Crociani, B.; di Bianca, F.; Giovenco, A.; Scrivanti, A. Protonation and methylation reactions of 2-pyridyl-palladium(II) and -platinum(II) complexes. J. Organomet. Chem. 1983, 251, 393–411. [Google Scholar] [CrossRef]
- Poulain, A.; Neels, A.; Albrecht, M. Palladium Complexes Containing Potentially Chelating Pyridylidene-Type Carbene Ligands. Eur. J. Inorg. Chem. 2009, 2009, 1871–1881. [Google Scholar] [CrossRef]
- Jahnke, M.C.; Pichl, R.M.C.; Hahn, F.E. Oxidative Addition of 2-Halogenopyridines and 2-Chloroquinoline to Zero-Valent Group 10 Metals. Z. Anorg. Allg. Chem. 2021, 647, 448–455. [Google Scholar] [CrossRef]
- Hervé, A.; Jahnke, M.C.; Pape, T.; Hahn, F.E. Synthesis of Palladium and Platinum Complexes with an NH, NEt-substituted Benzimidazolin-2-ylidene Ligand. Z. Anorg. Allg. Chem. 2013, 639, 2450–2454. [Google Scholar] [CrossRef]
- Das, R.; Hepp, A.; Daniliuc, C.G.; Hahn, F.E. Synthesis of Complexes with Protic NH,NH-NHC Ligands via Oxidative Addition of 2-Halogenoazoles to Zero-Valent Transition Metals. Organometallics 2014, 33, 6975–6987. [Google Scholar] [CrossRef]
- Jahnke, M.C.; Hervé, A.; Kampert, F.; Hahn, F.E. Synthesis of palladium complexes with anionic N,NR- or neutral NH,NR-theophylline-derived NHC ligands. Inorganica Chim. Acta 2021, 515, 120055. [Google Scholar] [CrossRef]
- Blumenberg, J.; Wilm, L.F.B.; Hahn, F.E. Synthesis of PdII Complexes Bearing pNHC Ligands by Oxidative Addition of 8-Halogenotheophyllines and 8-Bromoadenine to Pd0. Organometallics 2020, 39, 1281–1287. [Google Scholar] [CrossRef]
- Jahnke, M.C.; Mandal, S.; Kampert, F.; Tan, T.T.Y.; Müller, J.; Hahn, F.E. Oxidative addition of 8-bromo-9-ethyl-1,N6-ethenoadenine to d10 metals. Inorganica Chim. Acta 2023, 546, 121291. [Google Scholar] [CrossRef]
- Termühlen, S.; Blumenberg, J.; Hepp, A.; Daniliuc, C.G.; Hahn, F.E. Preparation of Complexes Bearing N-Alkylated, Anionic or Protic CAACs Through Oxidative Addition of 2-Halogenoindole Derivatives. Angew. Chem. Int. Ed. 2021, 60, 2599–2602. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Lonergan, D.; Perez, C.; Nammalwar, B.; Fan, J.; Martin, D.P.; Puerta, D.T. Substituted Piperidines and Substituted Tetrahydropyridines as Immune-Modulating Compounds. WO2023205173A1, 18 April 2023. [Google Scholar]
- Teng, M.; Puerta, D.T.; Lonergan, D.; Perez, C.; Nammalwar, B.; Martin, D.P. Glutaminyl-Peptide Cyclotransferase like (qpctl) Protein Inhibitors and Uses Thereof. WO2022086920A1, 19 October 2021. [Google Scholar]
- Chernyshev, V.M.; Khazipov, O.V.; Shevchenko, M.A.; Chernenko, A.Y.; Astakhov, A.V.; Eremin, D.B.; Pasyukov, D.V.; Kashin, A.S.; Ananikov, V.P. Revealing the unusual role of bases in activation/deactivation of catalytic systems: O-NHC coupling in M/NHC catalysis. Chem. Sci. 2018, 9, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Prototropic Tautomerism of Heterocycles: Heteroaromatic Tautomerism—General Overview and Methodology. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Amsterdam, The Netherlands, 2000; Volume 76, pp. 1–84. [Google Scholar]
- Ozimiński, W.P.; Dobrowolski, J.C.; Mazurek, A.P. DFT studies on tautomerism of C5-substituted 1,2,4-triazoles. J. Mol. Struct. Theochem 2004, 680, 107–115. [Google Scholar] [CrossRef]
- Pylypenko, O.O.; Okovytyy, S.I.; Sviatenko, L.K.; Voronkov, E.O.; Shabelnyk, K.P.; Kovalenko, S.I. Tautomeric behavior of 1,2,4-triazole derivatives: Combined spectroscopic and theoretical study. Struct. Chem. 2023, 34, 181–192. [Google Scholar] [CrossRef]
- Yamamuro, O.; Onoda-Yamamuro, N.; Matsuo, T.; Suga, H.; Kamiyama, T.; Ishigaki, T.-R.; Asano, H. X-ray and neutron diffraction studies of methylammonium tetrafluoroborate: Highly disordered orientations of CH3NH3+ and BF4− ions. J. Phys. Chem. Sol. 1995, 56, 183. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.44; Rigaku Oxford Diffraction: Abingdon, UK, 2025.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Atom | Length/Å | Atom | Angle/° |
|---|---|---|---|
| Pd(1)-Br(1) | 2.4721(3) | P(1)-Pd(1)-Br(1) | 91.532(16) |
| Pd(1)-P(1) | 2.3558(6) | P(2)-Pd(1)-Br(1) | 92.031(15) |
| Pd(1)-P(2) | 2.3308(6) | P(2)-Pd(1)-P(1) | 175.81(2) |
| Pd(1)-C(1) | 1.977(2) | C(1)-Pd(1)-Br(1) | 177.40(7) |
| C(1)-Pd(1)-P(1) | 88.20(7) | ||
| C(1)-Pd(1)-P(2) | 88.34(7) |
| D-H…A | d(D-H), Å | d(H…A), Å | d(D…A), Å | <(DHA)/° |
|---|---|---|---|---|
| N(1)-H(1)…F(4) | 0.88(3) | 2.03(3) | 2.872(3) | 159(3) |
| N(1)-H(1)…F(4A) | 0.88(3) | 1.79(3) | 2.622(11) | 158(3) |
| N(1)-H(1)…F(1B) | 0.88(3) | 1.92(4) | 2.72(3) | 150(3) |
| Parameter | Value |
|---|---|
| Empirical formula | C45H45BBrF4N3P2Pd |
| Formula weight | 962.90 |
| Temperature | 100.0(1) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions | a = 12.75750(10) Å |
| b = 24.1966(3) Å | |
| c = 13.2159(2) Å | |
| β = 93.4020(10)° | |
| Volume | 4072.40(9) Å3 |
| Z | 4 |
| Density (calculated) | 1.571 g/cm3 |
| Absorption coefficient | 1.571 mm−1 |
| F(000) | 1952 |
| Crystal size | 0.74 × 0.41 × 0.14 mm3 |
| θ range for data collection | 2.156 to 27.998° |
| Index ranges | −16 ≤ h ≤ 16, −31 ≤ k ≤ 31, −17 ≤ l ≤ 17 |
| Reflections collected | 58,724 |
| Independent reflections | 9841 [R(int) = 0.0381] |
| Observed reflections | 9111 |
| Completeness to θmax/θfull | 1.000/1.000 |
| Max./min. transmission | 1.00000/0.51526 |
| Data/restraints/parameters | 9841/166/551 |
| Goodness-of-fit on F2 | 1.033 |
| Final R indices [I>2σ(I)] | R1 = 0.0327, wR2 = 0.0887 |
| R indices (all data) | R1 = 0.0353, wR2 = 0.0903 |
| Largest diff. peak/hole | 1.900/−0.603 e·Å−3 |
| CCDC deposition number | 2497031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernenko, A.Y.; Lavrentev, I.V.; Shevchenko, M.A.; Minyaev, M.E.; Shepelenko, K.E.; Chernyshev, V.M. Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate. Molbank 2025, 2025, M2086. https://doi.org/10.3390/M2086
Chernenko AY, Lavrentev IV, Shevchenko MA, Minyaev ME, Shepelenko KE, Chernyshev VM. Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate. Molbank. 2025; 2025(4):M2086. https://doi.org/10.3390/M2086
Chicago/Turabian StyleChernenko, Andrey Y., Igor V. Lavrentev, Maxim A. Shevchenko, Mikhail E. Minyaev, Konstantin E. Shepelenko, and Victor M. Chernyshev. 2025. "Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate" Molbank 2025, no. 4: M2086. https://doi.org/10.3390/M2086
APA StyleChernenko, A. Y., Lavrentev, I. V., Shevchenko, M. A., Minyaev, M. E., Shepelenko, K. E., & Chernyshev, V. M. (2025). Bromo(5-cyclohexyl-1-methyl-1H-1,2,4-triazol-4-ium-3-yl)bis(triphenylphosphane)palladium Tetrafluoroborate. Molbank, 2025(4), M2086. https://doi.org/10.3390/M2086






