(E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline
Abstract
1. Introduction
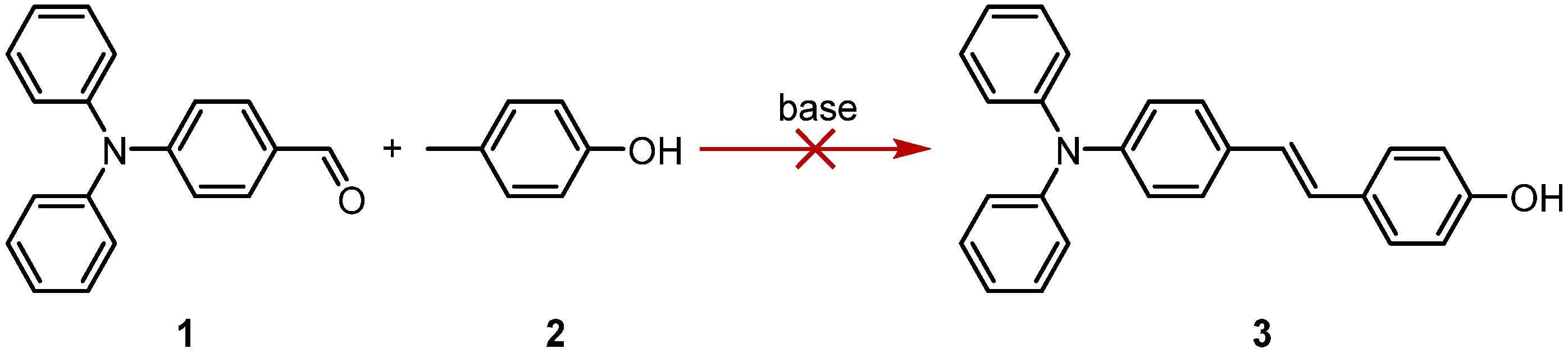
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Instrumentations
3.2. N,N-Diphenyl-4-vinylaniline (4)
3.3. (E)-4-(4-(Diphenylamino)styryl)phenol (3)
3.4. (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zheng, M.; Cai, J. Small molecules with membrane-active antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 21292–21299. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Yang, Y.; Cai, J.; Kong, H.; Bai, M.; Fu, X.; Qin, S.; Zhang, E. Synthesis and bioactivities of new membrane-active agents with aromatic linker: High selectivity and broad-spectrum antibacterial activity. ACS Infect. Dis. 2019, 5, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Chen, S.; Ampomah-Wireko, M.; Gao, C.; Xia, Z.; Nininahazwe, L.; Qin, S.; Zhang, E. Development of biaromatic core-linked antimicrobial peptide mimics: Substituent position significantly affects antibacterial activity and hemolytic toxicity. Eur. J. Med. Chem. 2022, 247, 115029. [Google Scholar] [CrossRef] [PubMed]
- Karak, A.; Manna, S.K.; Mahapatra, A.K. Triphenylamine-based small-molecule fluorescent probes. Anal. Methods 2022, 14, 972–1005. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhao, Z.; Cheng, D. Recent progress on triphenylamine materials: Synthesis, properties, and applications. Mol. Cryst. Liq. Cryst. 2017, 648, 223–235. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, Y.; Yang, X.; Ren, Y.; Liu, G.; Zhang, M.-X. Synthesis and properties of D-π-A triphenylamine derivatives with solvatochromism and bioimaging application. J. Photochem. Photobiol. A Chem. 2023, 444, 115002. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, D.; Yang, J.; Fang, M.; Li, Z. Tunable photoresponsive behaviors based on triphenylamine derivatives: The pivotal role of π-conjugated structure and corresponding application. Adv. Mater. 2021, 33, 2104002. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhou, C.; Wu, S.; Yu, B.; Zhang, Z.; Song, N.; Lee, M.M.S.; Xu, W.; Xu, F.-J.; Wang, D.; et al. Evaluation of structure-function relationships of aggregation-induced emission luminogens for simultaneous dual applications of specific discrimination and efficient photodynamic killing of gram-positive bacteria. J. Am. Chem. Soc. 2019, 141, 16781–16789. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.S.; Yu, E.Y.; Yan, D.; Chau, J.H.C.; Wu, Q.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Wang, D.; Tang, B.Z. The role of structural hydrophobicity on cationic amphiphilic aggregation-induced emission photosensitizer-bacterial interaction and photodynamic efficiency. ACS Nano 2023, 17, 17004–17020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Xia, F.-W.; Wang, Y.; Shi, H.-Z.; Wang, L.-J.; Zhao, Y.; Song, J.-X.; Wu, M.-Y.; Feng, S. Molecular charge and antibacterial performance relationships of aggregation-induced emission photosensitizers. ACS Appl. Mater. Interfaces 2023, 15, 17433–17443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhuang, Z.; Xing, L.; Li, J.; Yang, Z.; Ji, S.; Hu, R.; Zhao, Z.; Huo, Y.; Tang, B.Z. The AIE-active dual-cationic molecular engineering: Synergistic effect of dark toxicity and phototoxicity for anticancer therapy. Adv. Funct. Mater. 2021, 31, 2106988. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Zhuang, Z.; Wang, B.; Dai, J.; Feng, G.; Lou, X.; Xia, F.; Zhao, Z.; Tang, B.Z. Effective therapy of drug-resistant bacterial infection by killing planktonic bacteria and destructing biofilms with cationic photosensitizer based on phosphindole oxide. Small 2022, 18, 22007439. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, M.; Li, Z.-H.; Qu, Y.; Li, Y.; Ampomah-Wireko, M.; Li, D.; Kong, H.; Wu, Y.; Hossain, A.A.; et al. Important role of triphenylamine in modulating the antibacterial performance relationships of antimicrobial peptide mimics by alkyl chain engineering. J. Med. Chem. 2025, 68, 10299–10313. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-Q.; Wang, Y.-N.; Zhu, K.-W.; Li, R.; Ampomah-Wireko, M.; Amengor, C.D.K.; Zhang, E.; Zhao, Y.-H. (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline. Molbank 2025, 2025, M2087. https://doi.org/10.3390/M2087
Sun Y-Q, Wang Y-N, Zhu K-W, Li R, Ampomah-Wireko M, Amengor CDK, Zhang E, Zhao Y-H. (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline. Molbank. 2025; 2025(4):M2087. https://doi.org/10.3390/M2087
Chicago/Turabian StyleSun, Yi-Qiong, Ya-Na Wang, Kai-Wei Zhu, Ruirui Li, Maxwell Ampomah-Wireko, Cedric Dzidzor Kodjo Amengor, En Zhang, and Yi-Hong Zhao. 2025. "(E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline" Molbank 2025, no. 4: M2087. https://doi.org/10.3390/M2087
APA StyleSun, Y.-Q., Wang, Y.-N., Zhu, K.-W., Li, R., Ampomah-Wireko, M., Amengor, C. D. K., Zhang, E., & Zhao, Y.-H. (2025). (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline. Molbank, 2025(4), M2087. https://doi.org/10.3390/M2087





