Straightforward Synthesis of Thiophene Bioisosteres of the Pyrrolo[3,2-c]quinoline Framework from Martinelline Alkaloids
Abstract
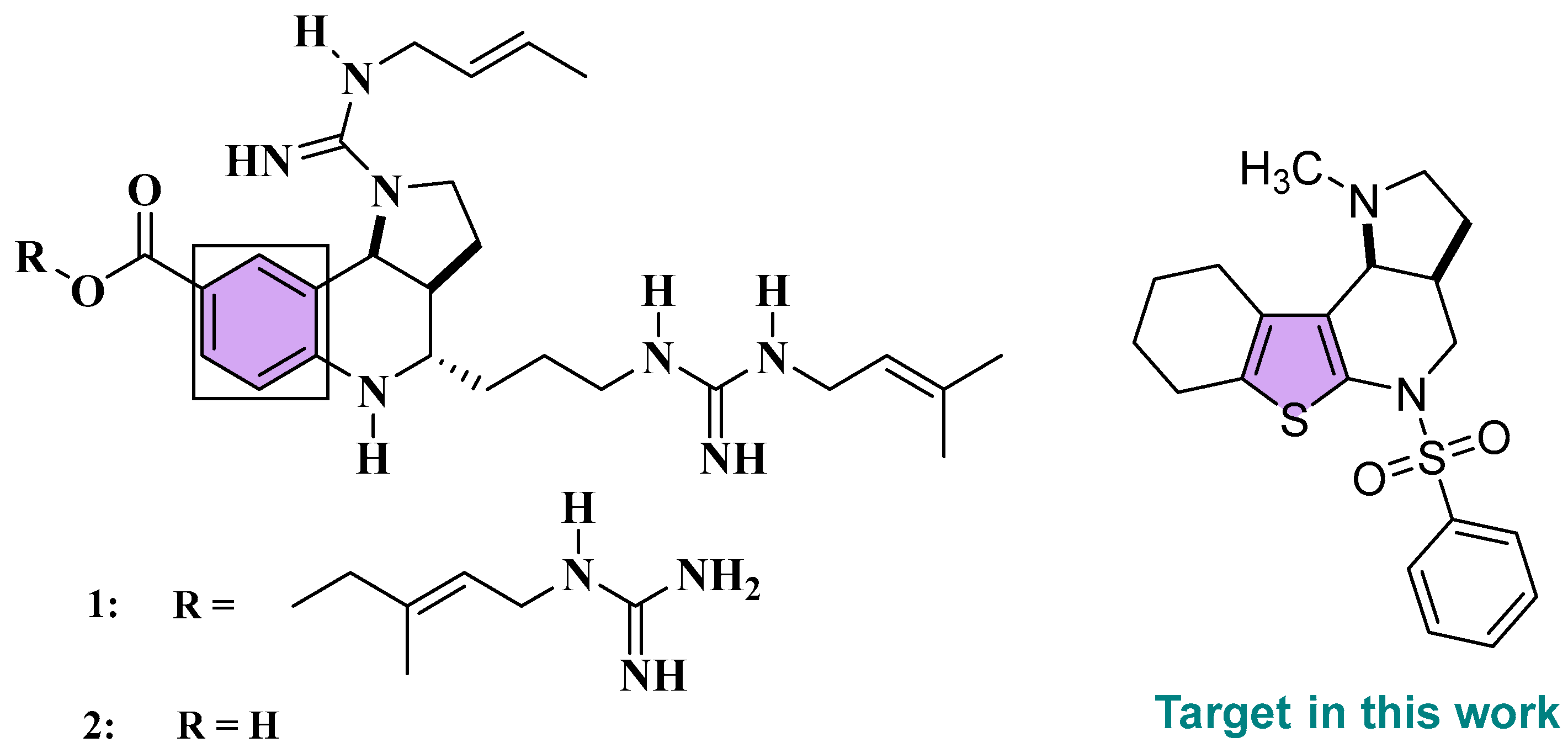
1. Introduction
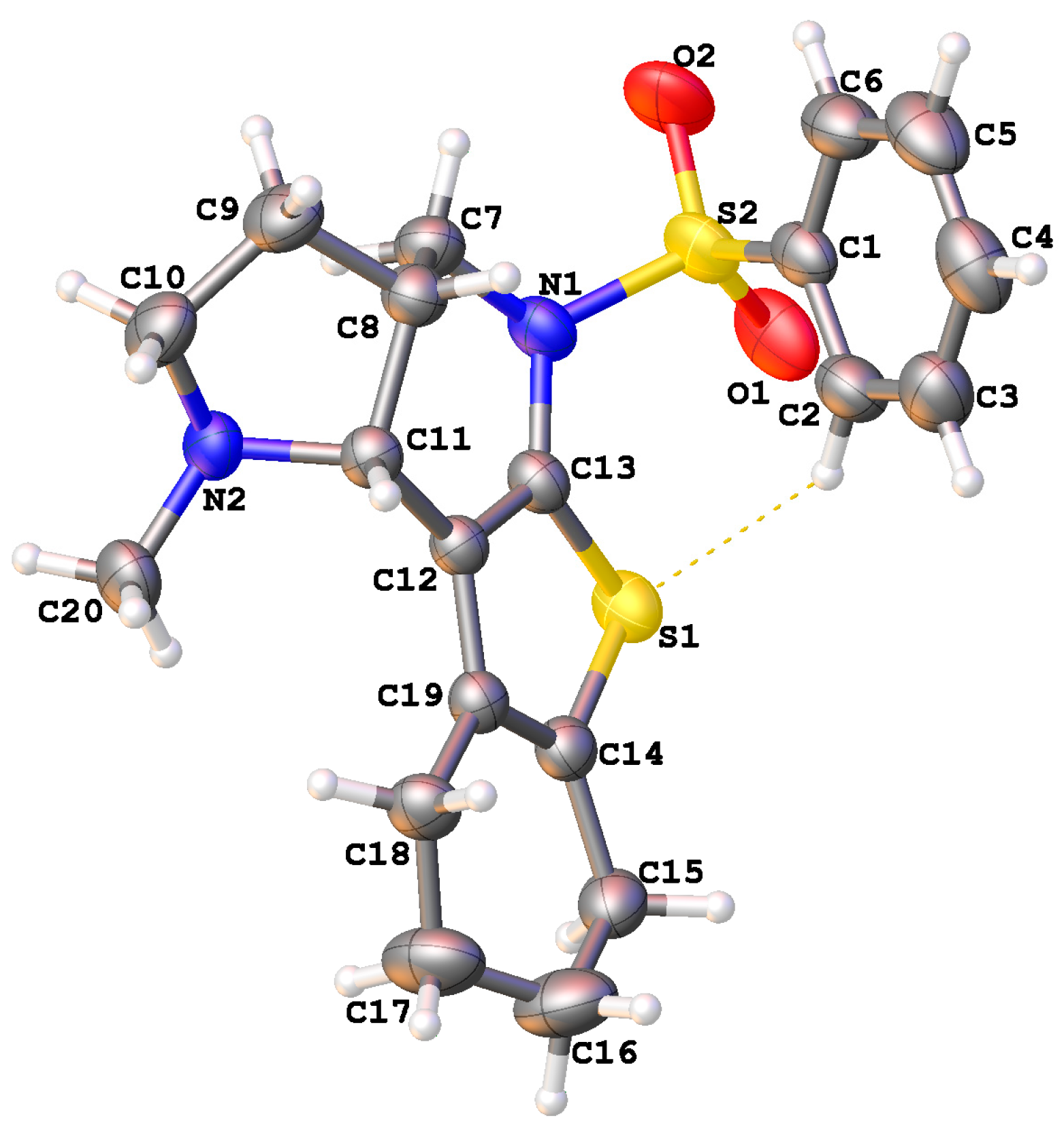
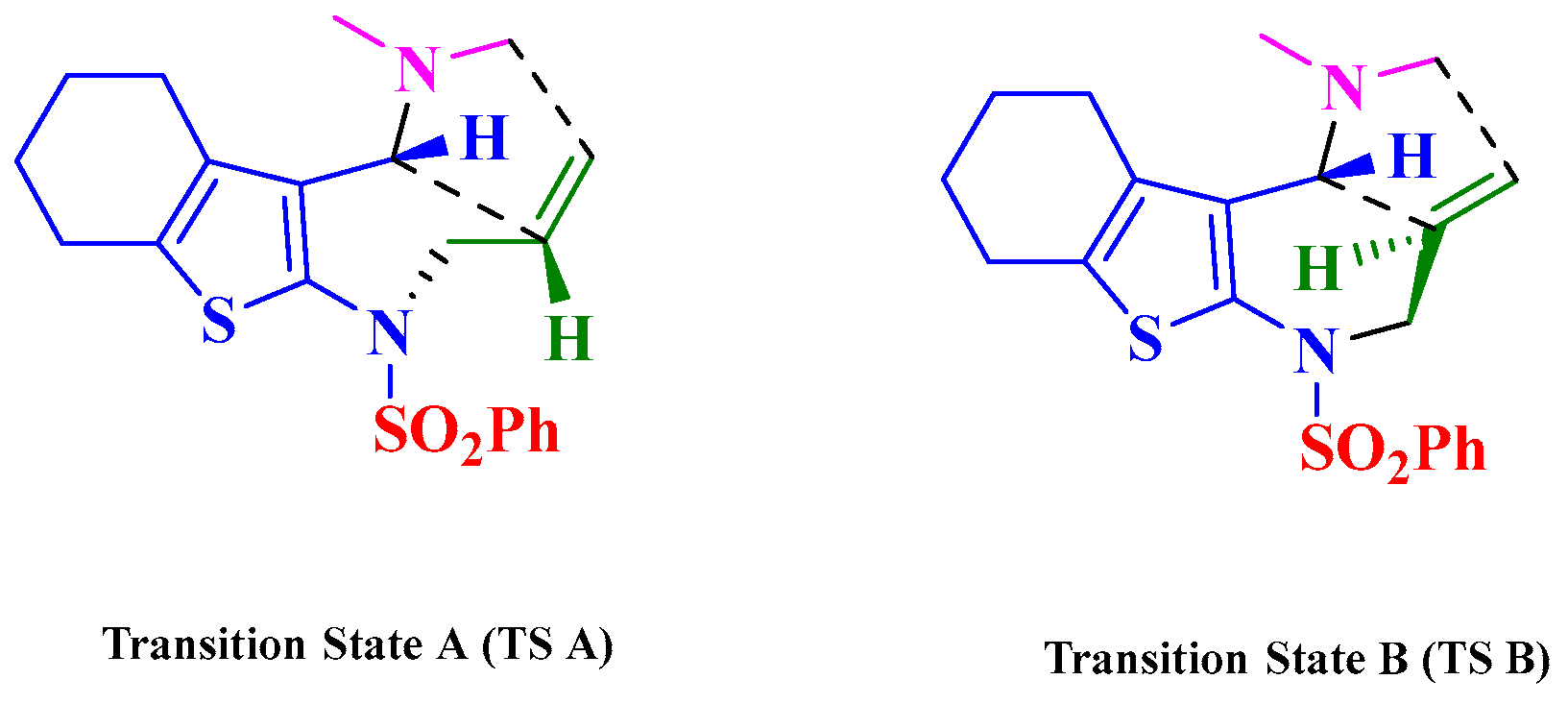
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Crystallographic Studies
3.3. Synthesis 1-Methyl-5-(phenylsulfonyl)-2,3,3a,4,5,7,8,9,10,10c-decahydro-1H-benzo[4,5]thieno[2,3-b]pyrrolo[2,3-d]pyridine (8)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordier, C.; Cooper, A.I. Natural products as an inspiration in the diversity-oriented synthesis of bioactive compound libraries. Nat. Prod. Rep. 2008, 25, 987–1009. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Tang, J.C.; Cheng, C.H.; Bian, Z.X.; Wong, W.Y.; Lam, K.H.; Chui, C.H. Synthesis of hexahydrofuro[3,2-c]quinoline, a martinelline type analogue with anticancer and antifungal activity. BMC Chem. 2016, 10, 9. [Google Scholar]
- Bro, F.S.; Laraia, L. Unifying principles for the design and evaluation of natural product-inspired compound collections. Chem. Sci. 2025, 16, 2961–2979. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Diversity-Oriented Synthesis and Chemoinformatics: A Fruitful Synergy towards Better Chemical Libraries. Eur. J. Org. Chem. 2022, 2022, e202200575. [Google Scholar] [CrossRef]
- Chen, L.; Chen, P.; Jia, Y. Bioinspired Total Synthesis of Natural Products. Acc. Chem. Res. 2024, 57, 3524–3540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Carl, J. Lovely, Hossen Mahmud, An approach to the pyrroloquinoline core of martinelline and martinellic acid. Tetrahedron Lett. 1999, 40, 2079–2082. [Google Scholar]
- Genet, M. Green Chemistry in Natural Product Discovery Sustainable Strategies for Drug Development. J. Pharmacogn. Nat. Prod. 2024, 10, 287. [Google Scholar]
- Salam, M.A.; Imdadulhaq, E.S.; Al-Romaizan, A.N.; Saleh, T.S.; Mostafa, M.M.M. Ultrasound-Assisted 1,3-Dipolar Cycloadditions Reaction Utilizing Ni-Mg-Fe LDH: A Green and Sustainable Perspective. Catalysts 2023, 13, 650. [Google Scholar] [CrossRef]
- Saleh, T.S.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.N.; Al-Thabaiti, S.A.; Mokhtar, M.J. Mg–Al hydrotalcite as an efficient catalyst for microwave assisted regioselective 1, 3-dipolar cycloaddition of nitrilimines with the enaminone derivatives: A green protocol. J. Mol. Catal. A Chem. 2013, 367, 12–22. [Google Scholar] [CrossRef]
- Sridhar, M.; Rao, R.M.; Baba, N.H.K.; Kumbhare, R.M. Microwave accelerated Gewald reaction: Synthesis of 2-aminothiophenes. Tetrahedron Lett. 2007, 48, 3171–3172. [Google Scholar] [CrossRef]
- Mokhtar, M.; Saleh, T.S.; Ahmed, N.S.; Al-Thabaiti, S.A.; Al-Shareef, R.A. An eco-friendly N-sulfonylation of amines using stable and reusable Zn–Al–hydrotalcite solid base catalyst under ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.; Lovely, C.J.; Dias, H.V.R. An intramolecular azomethine ylide–alkene cycloaddition approach to pyrrolo[3,2-c]quinolines-synthesis of a C2-truncated martinelline model. Tetrahedron 2001, 57, 4095–4105. [Google Scholar]
- Houk, K.N.; Paddon-Row, M.N.; Rondan, N.G.; Wu, Y.D.; Brown, F.K.; Spellmeyer, D.; Metz, J.T.; Li, Y.; Loncharich, R.J. Theory and modeling of stereoselective organic reactions. Science 1986, 231, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.S.J. A Correlation of Reaction Rates. Am. Chem. Soc. 1955, 77, 334–338. [Google Scholar] [CrossRef]
- Machado, I.V.; Dos Santos, J.R.N.; Januario, M.A.P.; Corrêa, A.G. Greener organic synthetic methods: Sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrason. Sonochem. 2021, 78, 105704. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083. [Google Scholar] [CrossRef] [PubMed]
- Loua, J.-D.; Xub, Z.-N. Solvent free oxidation of alcohols with manganese dioxide. Tetrahedron Lett. 2002, 43, 6149–6150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, T.S.; Al-Bogami, A.S. Straightforward Synthesis of Thiophene Bioisosteres of the Pyrrolo[3,2-c]quinoline Framework from Martinelline Alkaloids. Molbank 2025, 2025, M2084. https://doi.org/10.3390/M2084
Saleh TS, Al-Bogami AS. Straightforward Synthesis of Thiophene Bioisosteres of the Pyrrolo[3,2-c]quinoline Framework from Martinelline Alkaloids. Molbank. 2025; 2025(4):M2084. https://doi.org/10.3390/M2084
Chicago/Turabian StyleSaleh, Tamer S., and Abdullah S. Al-Bogami. 2025. "Straightforward Synthesis of Thiophene Bioisosteres of the Pyrrolo[3,2-c]quinoline Framework from Martinelline Alkaloids" Molbank 2025, no. 4: M2084. https://doi.org/10.3390/M2084
APA StyleSaleh, T. S., & Al-Bogami, A. S. (2025). Straightforward Synthesis of Thiophene Bioisosteres of the Pyrrolo[3,2-c]quinoline Framework from Martinelline Alkaloids. Molbank, 2025(4), M2084. https://doi.org/10.3390/M2084





