8,8′-Dichloro-2,2,2′,2′-tetraethyl-4,4′-bibenzo[1,3,6,2]dioxazastannocinylidene
Abstract
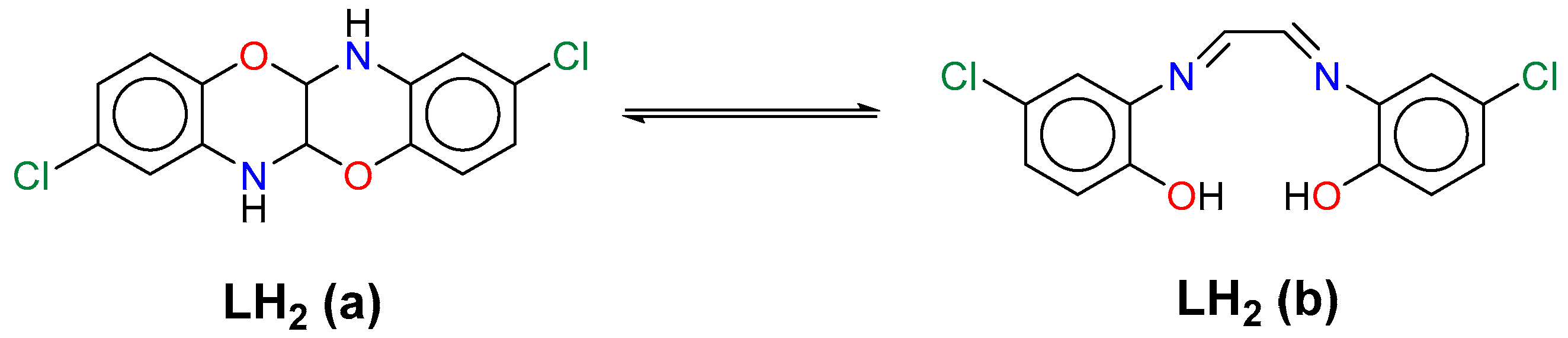
1. Introduction
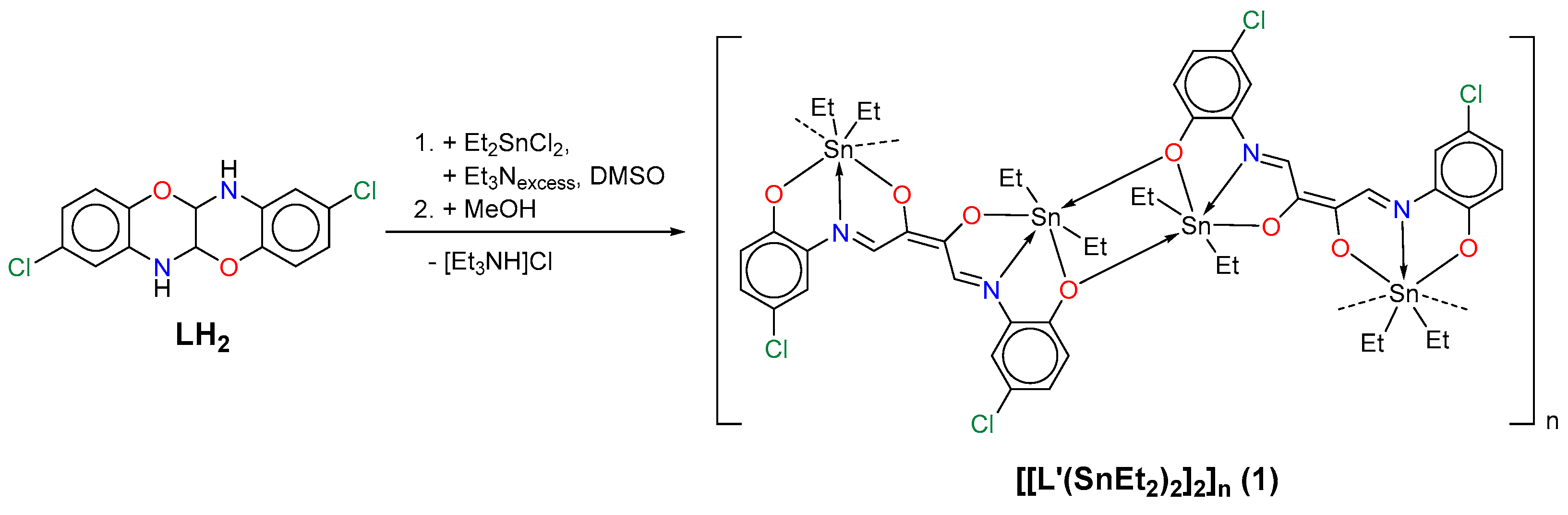
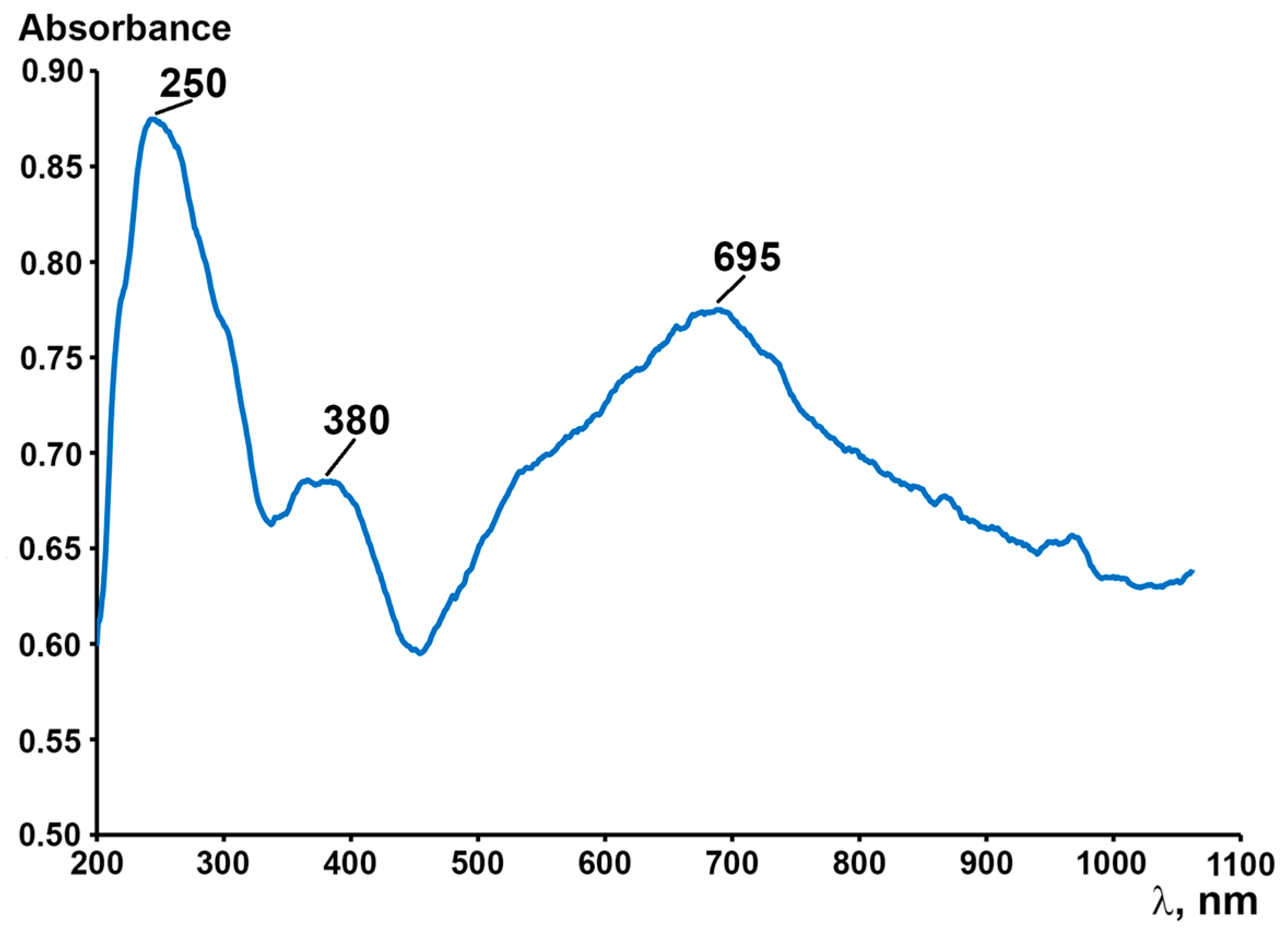
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of [[L’(SnEt2)2·DMSO]2]n (1)
3.3. Single-Crystal X-Ray Diffraction Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochoa, M.E.; Rojas-Lima, S.; Höpfl, H.; Rodríguez, P.; Castillo, D.; Farfán, N.; Santillan, R. Facile synthesis of 1,3,6-oxadiazepines from 2,2′-(1,2-ethanediyldiimino)bisphenols. Tetrahedron 2001, 57, 55–64. [Google Scholar] [CrossRef]
- Bandoli, G.; Clemente, D.A. Preparation and crystal structure of aqua[bis(2-hydroxyphenylimino)-ethanato-OO′NN′-]dioxouranium. J. Chem. Soc. Dalton Trans. 1975, 7, 612–615. [Google Scholar] [CrossRef]
- Min, K.S.; Weyhermüller, T.; Bothe, E.; Wieghardt, K. Tetradentate Bis(o-iminobenzosemiquinonate (1−)) π Radical Ligands and Their o-Aminophenolate (1−) Derivatives in Complexes of Nickel (II), Palladium (II), and Copper (II). Inorg. Chem. 2004, 43, 2922–2931. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Trofimova, O.Y.; Fukin, G.K.; Ketkov, S.Y.; Smolyaninov, I.V.; Cherkasov, V.K. Tin(IV) and lead(IV) complexes with a tetradentate redox-active ligand. Dalton Trans. 2012, 41, 10970–10979. [Google Scholar] [CrossRef]
- Takao, K.; Tsushima, S.; Ogura, T.; Tsubomura, T.; Ikeda, Y. Experimental and theoretical approaches to redox innocence of ligands in uranyl complexes: What is formal oxidation state of uranium in reductant of uranyl(VI)? Inorg. Chem. 2014, 53, 5772–5780. [Google Scholar] [CrossRef]
- Niklas, J.E.; Hunter, K.M.; Gorden, A.E. Bonding Interactions in Uranyl α-Diimine Complexes: A Spectroscopic and Electrochemical Study of the Impacts of Ligand Electronics and Extended Conjugation. Inorg. Chem. 2019, 58, 15088–15100. [Google Scholar] [CrossRef]
- Meshcheryakova, I.N.; Kocherova, T.N.; Yakushev, I.A.; Arseniev, M.V.; Starikov, A.G.; Kazakov, G.G.; Protasenko, N.A.; Piskunov, A.V. Features of the Molecular and Electronic Structures of the Vanadium(IV) Complex Based on Glyoxal-bis(2-hydroxy-3,5-di-tert-butylphenyl)Imine. J. Struct. Chem. 2025, 66, 489–501. [Google Scholar] [CrossRef]
- Lehtonen, A. Metal Complexes of Redox Non-Innocent Ligand N,N′-Bis(3,5-di-tertbutyl-2-hydroxy-phenyl)-1,2-phenylenediamine. Molecules 2024, 29, 1088. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakova, I.N.; Cherkasov, A.V.; Arsenyev, M.V.; Aysin, R.R.; Belikov, A.A.; Bogomyakov, A.S.; Protasenko, N.A.; Kocherova, T.N.; Baryshnikova, S.V.; Piskunov, A.V. Stepwise transformation of a redox-active tetradentate ONNO ligand in the coordination sphere of tin(IV). Dalton Trans. 2025, 54, 7281–7293. [Google Scholar] [CrossRef] [PubMed]
- Karnbrock, S.B.; Köster, J.F.; Becker, I.; Golz, C.; Meyer, F.; Gimferrer, M.; Alcarazo, M. Bis(amidophenolate)-supported pnictoranides: Lewis acid-induced electromerism in a bismuth complex. Chem. Sci. 2025, 16, 14178–14185. [Google Scholar] [CrossRef]
- Karnbrock, S.B.; Köster, J.F.; Golz, C.; Mata, R.A.; Alcarazo, M. Isolation of a Square Pyramidal Bis(amidophenolate)-Supported As(III)-Cation: Coordination-Induced Electromerism at As. Angew. Chem. Int. Ed. 2025, 64, e202501439. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Hess, M.; Müller, J.; Hildenbrand, K.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Aerobic oxidation of primary alcohols (including methanol) by Copper (II)− and Zinc (II)− phenoxyl radical catalysts. J. Am. Chem. Soc. 1999, 121, 9599–9610. [Google Scholar] [CrossRef]
- Blackmore, K.J.; Lal, N.; Ziller, J.W.; Heyduk, A.F. Catalytic reactivity of a zirconium(IV) redox-active ligand complex with 1,2-diphenylhydrazine. J. Am. Chem. Soc. 2008, 130, 2728–2729. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, A.L.; Kopilov, J.; Goldberg, I.; Coates, G.W.; Kol, M. New facets of an old ligand: Titanium and zirconium complexes of phenylenediamine bis(phenolate) in lactide polymerisation catalysis. Chem. Commun. 2009, 44, 6804–6806. [Google Scholar] [CrossRef]
- Tauer, E.; Grellmann, K.H.; Kaufmann, E.; Noltemeyer, M. The condensation product of 2-aminophenol and glyoxal. Structure and photochemistry. Chem. Berichte 1986, 119, 3316–3325. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Druzhkov, N.O.; Kurskii, Y.A.; Abakumova, L.G.; Shavyrin, A.S.; Fukin, G.K.; Poddel’skii, A.I.; Cherkasov, V.K.; Okhlopkova, L.S. Quinone imines and aminophenols as precursors of new heterocycles. Russ. Chem. Bull. 2005, 54, 2571–2577. [Google Scholar] [CrossRef]
- Bakaev, I.V.; Romashev, N.F.; Komlyagina, V.I.; Abramov, P.A.; Piskunov, A.V.; Gushchin, A.L. Palladium(II) complex with tetrahydrobenzoxazinobenzoxosine: Synthesis, electronic and molecular structures. J. Struct. Chem. 2022, 63, 1963–1972. [Google Scholar] [CrossRef]
- Camacho-Camacho, C.; Esparza-Ruiz, A.; Vásquez-Badillo, A.; Nöth, H.; Flores-Parra, A.; Contreras, R. Fused hexacyclic tin compounds derived from 3-(3,5-di-t-butyl-2-hydroxy-phenylimino)-3H-phenoxazin-2-ol. J. Organomet. Chem. 2009, 694, 726–730. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Poddel’sky, A.I.; Bellan, E.V.; Smolyaninov, I.V.; Cherkasov, A.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Ferrocene-Containing Tin(IV) Complexes Based on o-Benzoquinone and o-Iminobenzoquinone Ligands. Synthesis, Molecular Structure, and Electrochemical Properties. Inorg. Chem. 2020, 59, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Bruzeguini, C.E.T.; Bezerra, R.I.; Ribeiro, M.A. Synthesis and structure analysis of a cobalt(III) coordination compound obtained from a redox-active phenolate ligand and cobalt(II). Cryst. Struct. Commun. 2025, 81, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1980. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA diffraction beamline for studying crystalline samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 22 September 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheryakova, I.N.; Yakushev, I.A.; Cherkasov, A.V.; Arsenyev, M.V.; Klimashevskaya, A.V.; Kolevatov, D.S.; Piskunov, A.V. 8,8′-Dichloro-2,2,2′,2′-tetraethyl-4,4′-bibenzo[1,3,6,2]dioxazastannocinylidene. Molbank 2025, 2025, M2083. https://doi.org/10.3390/M2083
Meshcheryakova IN, Yakushev IA, Cherkasov AV, Arsenyev MV, Klimashevskaya AV, Kolevatov DS, Piskunov AV. 8,8′-Dichloro-2,2,2′,2′-tetraethyl-4,4′-bibenzo[1,3,6,2]dioxazastannocinylidene. Molbank. 2025; 2025(4):M2083. https://doi.org/10.3390/M2083
Chicago/Turabian StyleMeshcheryakova, Irina N., Ilya A. Yakushev, Anton V. Cherkasov, Maxim V. Arsenyev, Anastasiya V. Klimashevskaya, Dmitriy S. Kolevatov, and Alexandr V. Piskunov. 2025. "8,8′-Dichloro-2,2,2′,2′-tetraethyl-4,4′-bibenzo[1,3,6,2]dioxazastannocinylidene" Molbank 2025, no. 4: M2083. https://doi.org/10.3390/M2083
APA StyleMeshcheryakova, I. N., Yakushev, I. A., Cherkasov, A. V., Arsenyev, M. V., Klimashevskaya, A. V., Kolevatov, D. S., & Piskunov, A. V. (2025). 8,8′-Dichloro-2,2,2′,2′-tetraethyl-4,4′-bibenzo[1,3,6,2]dioxazastannocinylidene. Molbank, 2025(4), M2083. https://doi.org/10.3390/M2083





