2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate
Abstract
1. Introduction
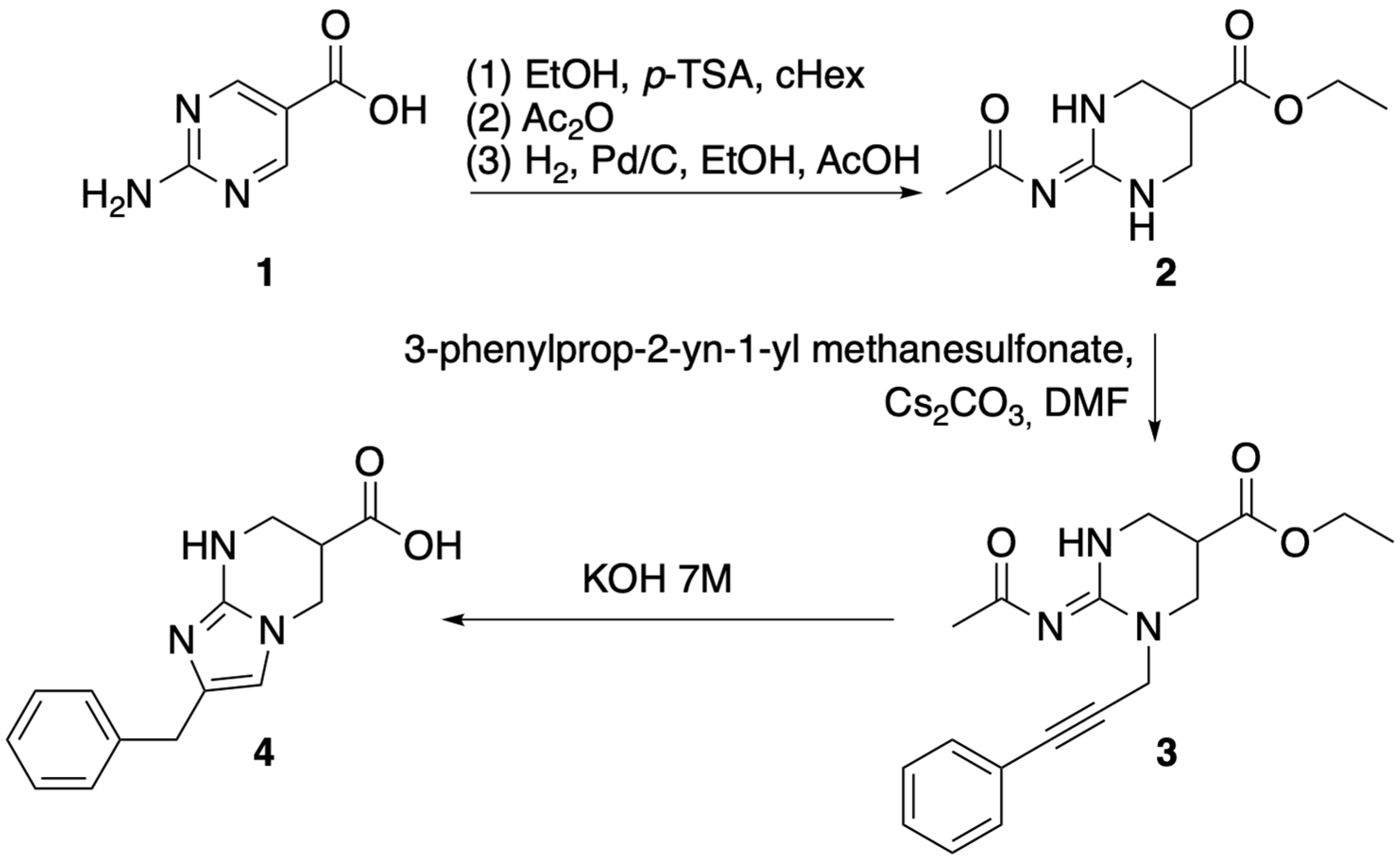
2. Results and Discussion
3. Materials and Methods
- Ethyl (E)-2-(acetylimino)-1-(3-phenylprop-2-yn-1-yl)hexahydropyrimidine-5-carboxylate (3). To a flame-dried two-neck flask, compound 2 (300 mg, 1.41 mmol, 1 eq) was added in dry DMF. Cs2CO3 (3 eq) was then added, and the suspension was stirred for 30 min under a nitrogen atmosphere. The alkylating agent 3-phenyl-2-propyn-1-yl methanesulfonate (350 mg, 1.7 mmol, 1.2 eq) was added, and the reaction mixture was stirred at 70 °C. After 48, the mixture was diluted with water in a 10-fold volume of the DMF, followed by 3 extractions with EtOAc. The combined organic layers were then washed with brine, dried over Na2SO4, filtered, and evaporated to dryness under vacuum. FC (Heptane/EtOAc) gave the pure compound 3 in 43% yield as a white solid. 1H NMR (600 MHz, DMSO-d6) δ 10.30 (t, J = 2.9 Hz, 1H), 7.49–7.42 (m, 2H), 7.42–7.34 (m, 3H), 4.73 (d, J = 17.5 Hz, 1H), 4.63 (d, J = 17.5 Hz, 1H), 4.10 (m, 2H), 3.67–3.60 (m, 2H), 3.56–3.51 (m, 1H), 3.49–3.44 (m, 1H), 3.19–3.12 (m, 1H), 1.90 (s, 3H), 1.16 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, DMSO) δ 181.52, 170.42, 156.35, 131.48, 128.67, 128.65, 122.08, 85.16, 83.19, 60.74, 44.74, 39.20, 37.36, 35.74, 28.18, 13.94. LC-MS (ESI): m/z calculated for C18H21N3O3 [M + H]+: 328.2, found: 328.2, retention time: 3.240 min.
- 2-benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate (4). Compound 3 (65 mg, 0.2 mmol) was stirred in 7M KOHaq at 60 °C for 3 h. Purification by preparative HPLC (20 min, 0% B to 80% B) provided the pure compound 4 (TFA salt) in 35% yield as an off-white solid. 1H NMR (600 MHz, DMSO-d6) δ 13.03 (broad s, 1H), 12.45 (broad s, 1H), 8.31 (t, J = 2.9 Hz, 1H), 7.36–7.31 (m, 2H), 7.27–7.22 (m, 3H), 6.77 (s, 1H), 4.05 (dd, J = 12.7, 5.7 Hz, 1H), 4.02 (dd, J = 12.7, 5.1 Hz, 1H), 3.80 (m, 2H), 3.56–3.49 (m, 2H), 3.25–3.19 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.63, 157.90 (q, JCF = 30.3 Hz), 143.68, 137.49, 128.54, 128.48, 126.72, 125.67, 117.31 (q, JCF = 300.9 Hz), 112.62, 43.24, 39.73, 35.37, 30.06. LC-MS (ESI): m/z calculated for C14H15N3O2 [M + H]+: 258.1, found: 258.1. Retention time: 2.744 min. HRMS (ESI): m/z calculated for C14H15N3O2 [M + H]+: 258.1238, found: 258.1242. Purity by analytical HPLC (210 nm): 95%.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; De, S.; Chen, C. Syntheses of cyclic guanidine-containing natural products. Tetrahedron 2015, 71, 1145–1173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef] [PubMed]
- Al-Taie, Z.S.; Bartholomew, B.; Coles, S.J.; Evans, D.M.; Hollinshead, J.; Jones, L.F.; Kraehenbuehl, R.; Murphy, P.J.; Nash, R.J.; Penkova, Y.B.; et al. Cyclic guanidine containing amino acids that promote glucocerebrosidase. Tetrahedron 2022, 123, 132959. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.-W.; Guo, Y.-W. Recent Advances in the Isolation, Synthesis and Biological Activity of Marine Guanidine Alkaloids. Mar. Drugs 2017, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Varela, C.L.; Pires, A.S.; Tavares-da-Silva, E.J.; Roleira, F.M.F. Synthetic and natural guanidine derivatives as antitumor and antimicrobial agents: A review. Bioorgan. Chem. 2023, 138, 106600. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.J.; Naysmith, B.J.; Furkert, D.P.; Brimble, M.A. Enduracididine, a rare amino acid component of peptide antibiotics: Natural products and synthesis. Beilstein J. Org. Chem. 2016, 12, 2325–2342. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Cheng, T.; Wang, Y.-L.; Huang, S.-J.; Hsiao, Y.-H.; Lai, Y.-T.; Toh, S.-I.; Chu, J.; Rudolf, J.D.; Chang, C.-Y. Characterization and Structural Determination of CmnG-A, the Adenylation Domain That Activates the Nonproteinogenic Amino Acid Capreomycidine in Capreomycin Biosynthesis. ChemBioChem 2022, 23, e202200563. [Google Scholar] [CrossRef] [PubMed]
- Kickinger, S.; Al-Khawaja, A.; Haugaard, A.S.; Lie, M.E.K.; Bavo, F.; Löffler, R.; Damgaard, M.; Ecker, G.F.; Frølund, B.; Wellendorph, P. Exploring the molecular determinants for subtype-selectivity of 2-amino-1,4,5,6-tetrahydropyrimidine-5-carboxylic acid analogs as betaine/GABA transporter 1 (BGT1) substrate-inhibitors. Sci. Rep. 2020, 10, 12992. [Google Scholar] [CrossRef] [PubMed]
- Bolchi, C.; Bavo, F.; Pallavicini, M. One-step preparation of enantiopure l- or d-amino acid benzyl esters avoiding the use of banned solvents. Amino Acids 2017, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, M.; Laufer, S.A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62, 5673–5724. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, L.; Olesen, P.H.; Hansen, L.; Sheardown, M.J.; Thomsen, C.; Rasmussen, T.; Jensen, A.F.; Christensen, M.S.; Rimvall, K.; Ward, J.S.; et al. 1-(1,2,5-Thiadiazol-4-yl)-4-azatricyclo[2.2.1.02,6]heptanes as New Potent Muscarinic M1 Agonists: Structure−Activity Relationship for 3-Aryl-2-propyn-1-yloxy and 3-Aryl-2-propyn-1-ylthio Derivatives. J. Med. Chem. 1999, 42, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, T. Chiral Carboxylic Acids as Organic Catalysts for Asymmetric Reactions. ChemistrySelect 2023, 8, e202300010. [Google Scholar] [CrossRef]
- Bachrach, S.M. Chiral Superbases with Extended Hydrogen Bond Networks. J. Org. Chem. 2019, 84, 3467–3476. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bavo, F.; Avgerinos, C.; Martino, E.; Frølund, B. 2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate. Molbank 2025, 2025, M2077. https://doi.org/10.3390/M2077
Bavo F, Avgerinos C, Martino E, Frølund B. 2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate. Molbank. 2025; 2025(4):M2077. https://doi.org/10.3390/M2077
Chicago/Turabian StyleBavo, Francesco, Christos Avgerinos, Elena Martino, and Bente Frølund. 2025. "2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate" Molbank 2025, no. 4: M2077. https://doi.org/10.3390/M2077
APA StyleBavo, F., Avgerinos, C., Martino, E., & Frølund, B. (2025). 2-Benzyl-6-carboxy-5,6,7,8-tetrahydroimidazo[1,2-a]pyrimidin-1-ium 2,2,2-trifluoroacetate. Molbank, 2025(4), M2077. https://doi.org/10.3390/M2077





