N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide
Abstract
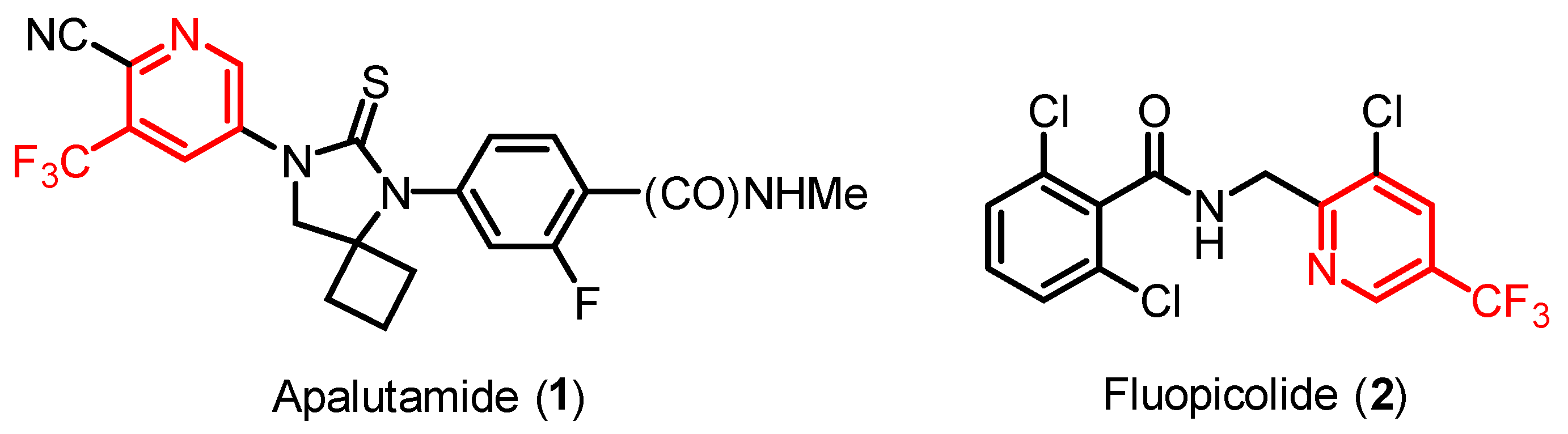
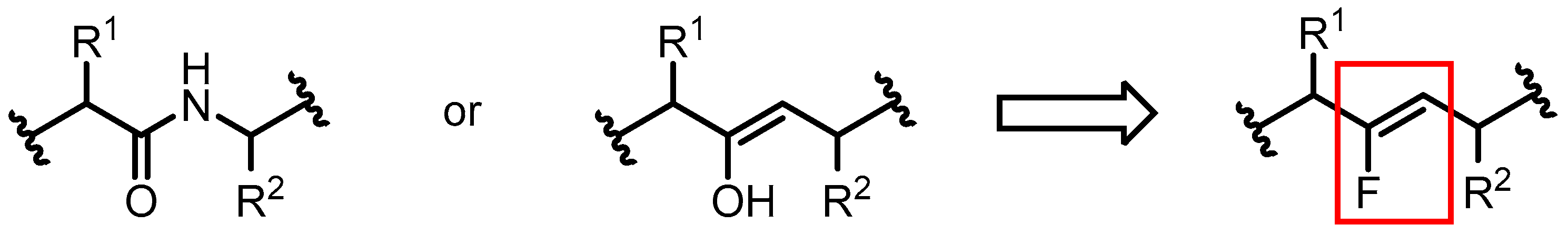
1. Introduction
2. Results
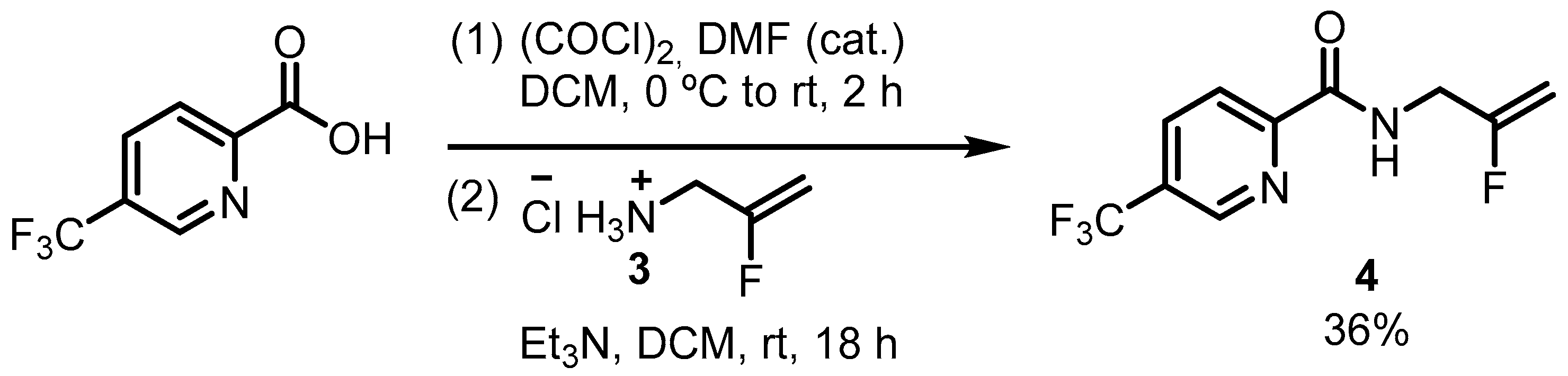
2.1. Synthesis and Spectroscopy
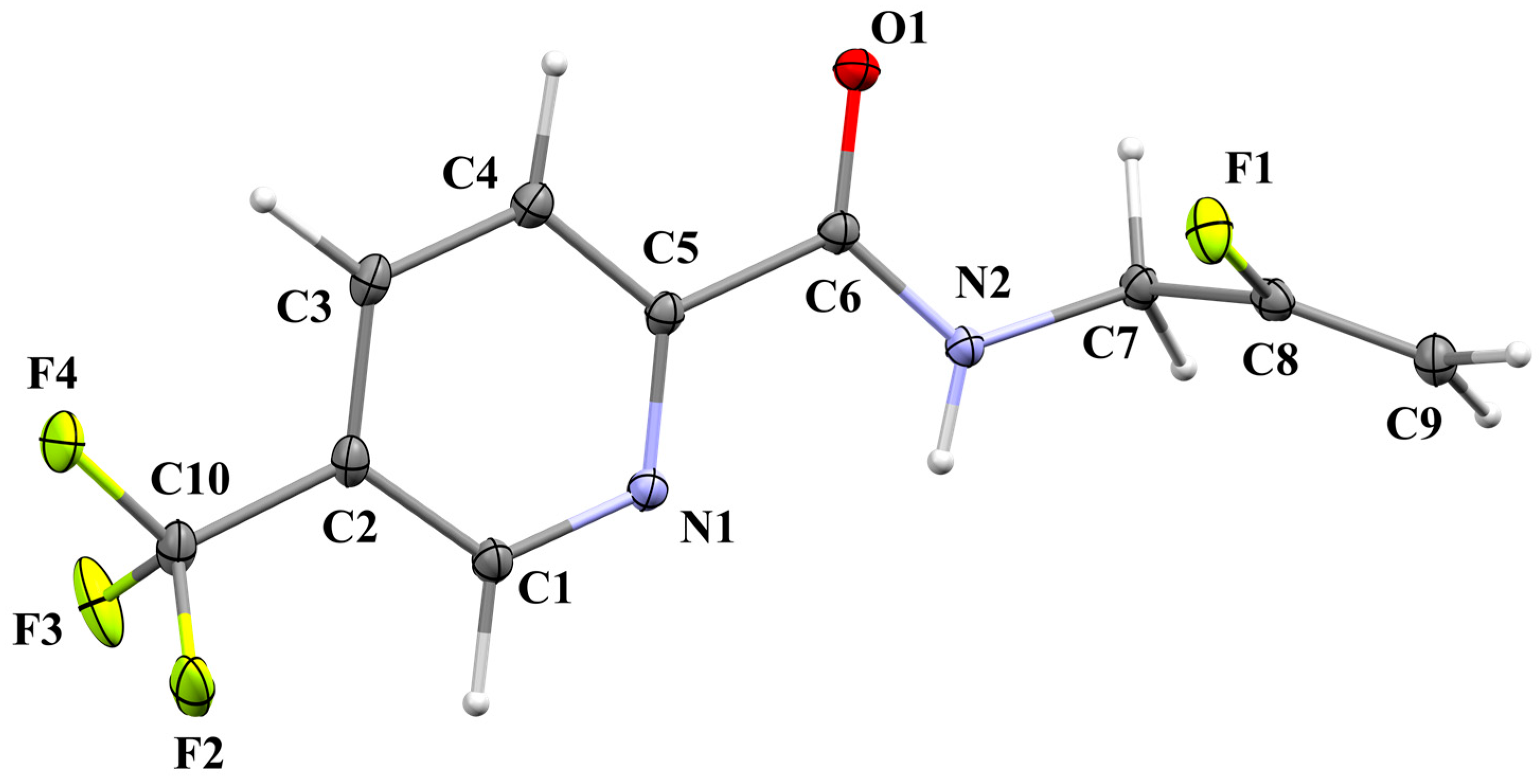
2.2. Crystal Structure of 4
2.2.1. Structural Commentary
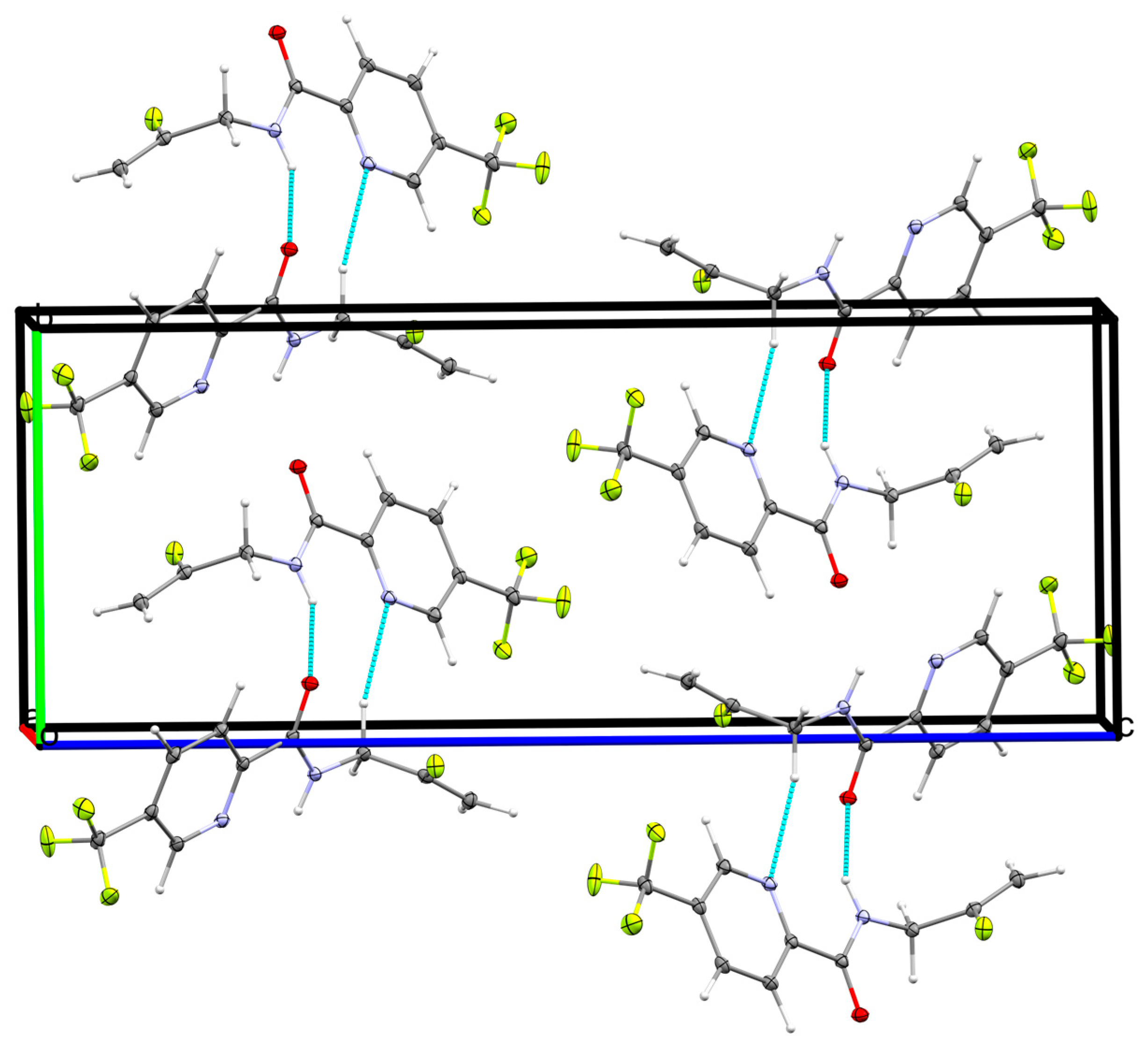
2.2.2. Supramolecular Features
2.2.3. Database Surve
3. Experimental Section
3.1. General Considerations
3.2. Synthesis of N-(2-fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide (4)
3.3. X-Ray Diffraction Data of 4
3.4. X-Ray Structure Refinement of 4
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Dcalc | Calculated density |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| FDA | Food and Drug Administration |
| H-bonding | Hydrogen bonding |
| HRMS | High-resolution mass spectrometry |
| IR | Infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| SC-XRD | Single-crystal X-ray diffraction |
| su | Standard uncertainty from least-squares refinement |
| VdW | Van der Waals |
References
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Champagne, P.A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef]
- Le Bars, D. Fluorine-18 and medical imaging: Radiopharmaceuticals for positron emission tomography. J. Fluorine Chem. 2006, 127, 1488–1493. [Google Scholar] [CrossRef]
- Nair, A.S.; Singh, A.K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-Approved Trifluoromethyl Group-Containing Drugs: A Review of 20 Years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Burriss, A.; Edmunds, A.J.; Emery, D.; Hall, R.G.; Jacob, O.; Schaetzer, J. The importance of trifluoromethyl pyridines in crop protection. Pest Manag. Sci. 2018, 74, 1228–1238. [Google Scholar] [CrossRef]
- Lu, M.-Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.-S.; Loh, T.-P. Recent Advances in Alkenyl sp2 C–H and C–F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479–17646. [Google Scholar] [CrossRef] [PubMed]
- Drouin, M.; Laxio Arenas, J.; Paquin, J.-F. Incorporating a Monofluoroalkene into the Backbones of Short Peptides: Evaluating the Impact on Local Hydrophobicity. ChemBioChem 2019, 20, 1817–1826. [Google Scholar] [CrossRef]
- Semeniuk, T.; Challenger, J.; Dudas, T.; Toporkov, D.; van Hoeve, M.D.; Hamel, J.-D. Photoredox Decarboxylative 3-Fluoroallylation of α-Amino Acids. Eur. J. Org. Chem. 2025, 28, e202500519. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Cheng, Y.-M.; Zhao, X.-W.; Cao, Z.-Y.; Xiao, X.; Xu, Y. Catalytic asymmetric synthesis of monofluoroalkenes and gem-difluoroalkenes: Advances and perspectives. Org. Chem. Front. 2021, 8, 2315–2327. [Google Scholar] [CrossRef]
- Brown, D.G.; Boström, J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2016, 59, 4443–4458. [Google Scholar] [CrossRef]
- Ameram, N.; Adam, F.; Fatihah, N.N.; Al-Juaid, S. Crystal structure of 2-methyl-N-[(4-methylpyridin-2-yl)carbamothioyl]-benzamide. Acta Crystallogr. E. 2015, 71, o356. [Google Scholar] [CrossRef]
- Dey, S.; Manogaran, D.; Manogaran, S.; Schaefer, H.F. Substituent effects on the aromaticity of benzene—An approach based on interaction coordinates. J. Chem. Phys. 2019, 150, 214108. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.A.K.; Hoy, V.J.; O’Hagan, D.; Smith, G.T. How good is fluorine as a hydrogen bond acceptor? Tetrahedron 1996, 52, 12613. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Panini, P.; Chopra, D. Quantitative insights into energy contributions of intermolecular interactions in fluorine and trifluoromethyl substituted isomeric N-phenylacetamides and N-methylbenzamides. CrystEngComm 2013, 15, 3711–3733. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Yang, B.; Fan, R. Imino exchange reaction in a dearomatization strategy: Synthesis of N-acyl diarylamines and phenothiazines from two anilines. Org. Chem. Front. 2014, 1, 1055–1057. [Google Scholar] [CrossRef]
- Light, M.E.; Tesfatsion, B.; Kilburn, J.; Dixon, S. CSD Communication, 2016; CCDC 1476136.
- Hernández-Gil, J.; Ferrer, S.; Ballesteros, R.; Castiñeiras, A. N-(5-Amino-1H-1,2,4-triazol-3-yl)-pyridine-2-carboxamide. Acta Crystallogr. E. 2013, 69, o227. [Google Scholar] [CrossRef]
- Villiers, E.; Couve-Bonnaire, S.; Cahard, D.; Pannecoucke, X. The fluoroalkene motif as a surrogate of the amide bond: Syntheses of AA-Ψ[(Z) and (E)-CFCH]-Pro pseudodipeptides and an Enalapril analogue. Tetrahedron 2015, 71, 7054–7062. [Google Scholar] [CrossRef]
- Nadon, J.-F.; Rochon, K.; Grastilleur, S.; Langlois, G.; Dao, T.T.H.; Blais, V.; Guérin, B.; Gendron, L.; Dory, Y.L. Synthesis of Gly-ψ[(Z)CF=CH]-Phe, a Fluoroalkene Dipeptide Isostere, and Its Incorporation into a Leu-enkephalin Peptidomimetic. ACS Chem. Neurosci. 2017, 8, 40–49. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, N.; Ma, X.; Nie, J.; Zhang, F.-G.; Ma, J.-A. Silver-Catalyzed [3 + 3] Dipolar Cycloaddition of Trifluorodiazoethane and Glycine Imines: Access to Highly Functionalized Trifluoromethyl-Substituted Triazines and Pyridines. ACS Catal. 2019, 9, 4600–4608. [Google Scholar] [CrossRef]
- CrysAlisPro; v1.171.42.94a & 109a; Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
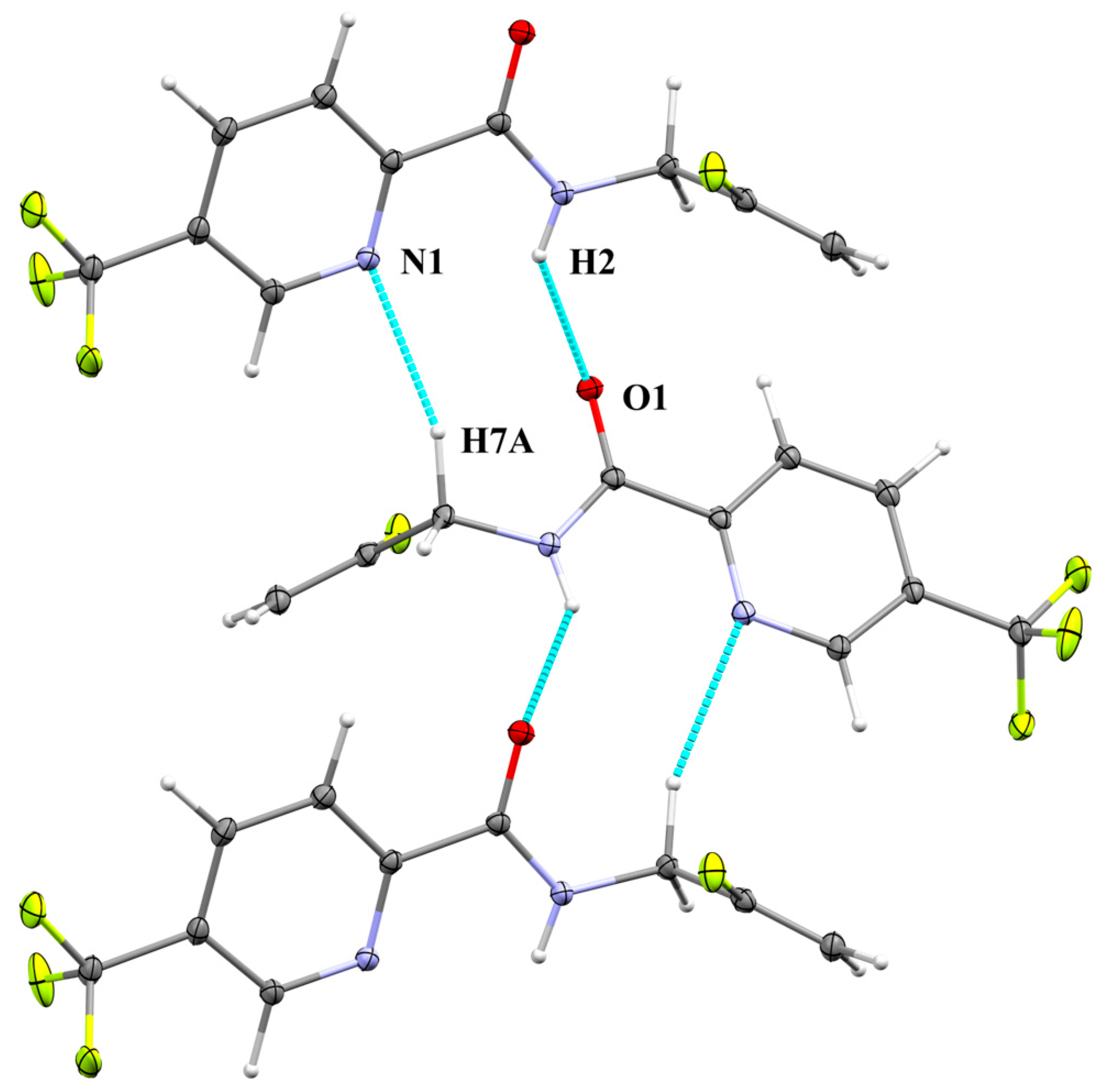
| Atoms | Length | Length—VdW | Symmetry Operation |
|---|---|---|---|
| N1···H7A | 2.464 | −0.396 | ½ − x, −½ + y, ½ − z |
| H2···O1 | 2.123 | −0.577 | ½ − x, −½ + y, ½ − z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeniuk, T.; Hamel, J.-D. N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide. Molbank 2025, 2025, M2078. https://doi.org/10.3390/M2078
Semeniuk T, Hamel J-D. N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide. Molbank. 2025; 2025(4):M2078. https://doi.org/10.3390/M2078
Chicago/Turabian StyleSemeniuk, Taylor, and Jean-Denys Hamel. 2025. "N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide" Molbank 2025, no. 4: M2078. https://doi.org/10.3390/M2078
APA StyleSemeniuk, T., & Hamel, J.-D. (2025). N-(2-Fluoro-2-propen-1-yl)-5-(trifluoromethyl)-2-pyridinecarboxamide. Molbank, 2025(4), M2078. https://doi.org/10.3390/M2078





