Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization
Abstract
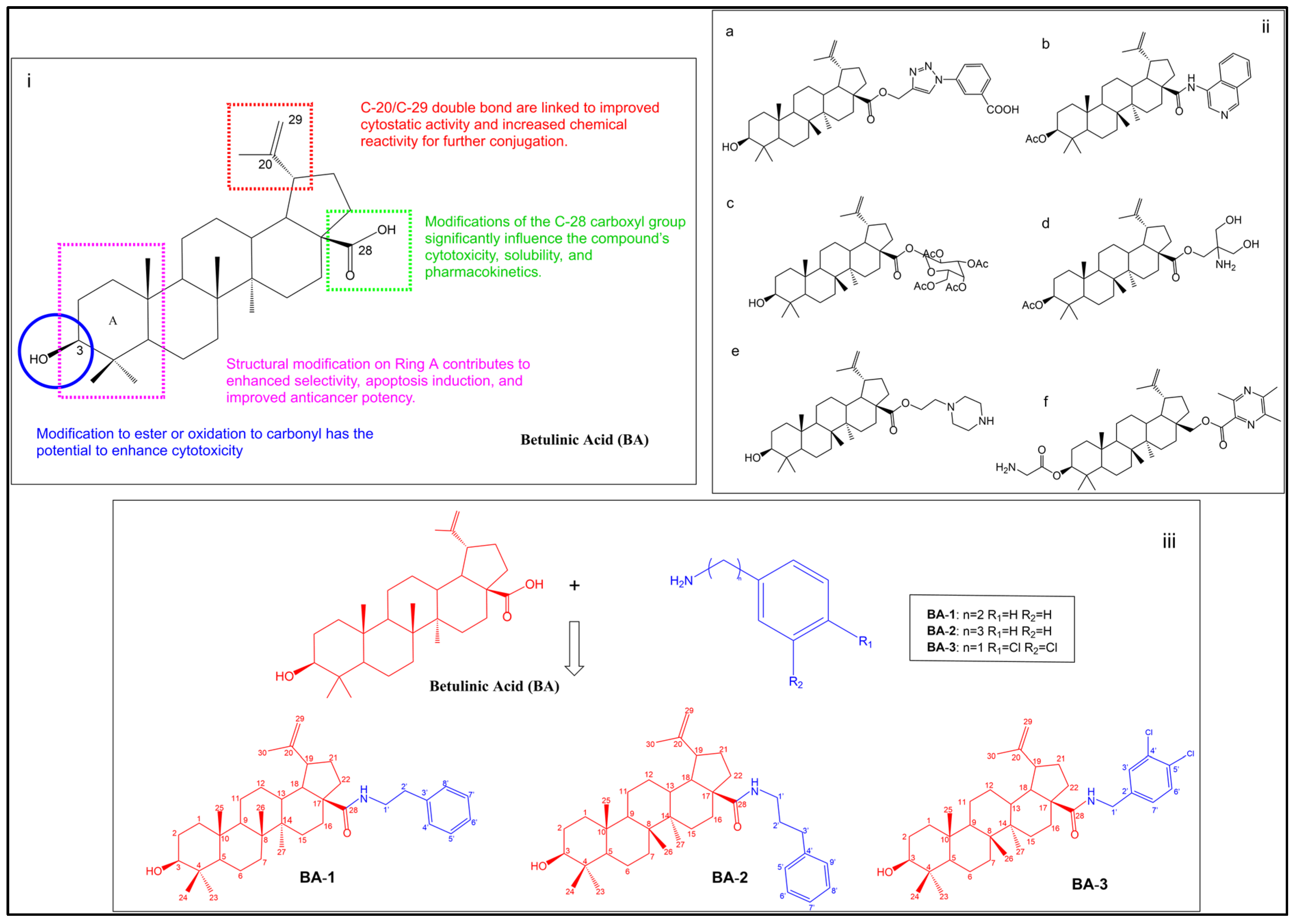
1. Introduction
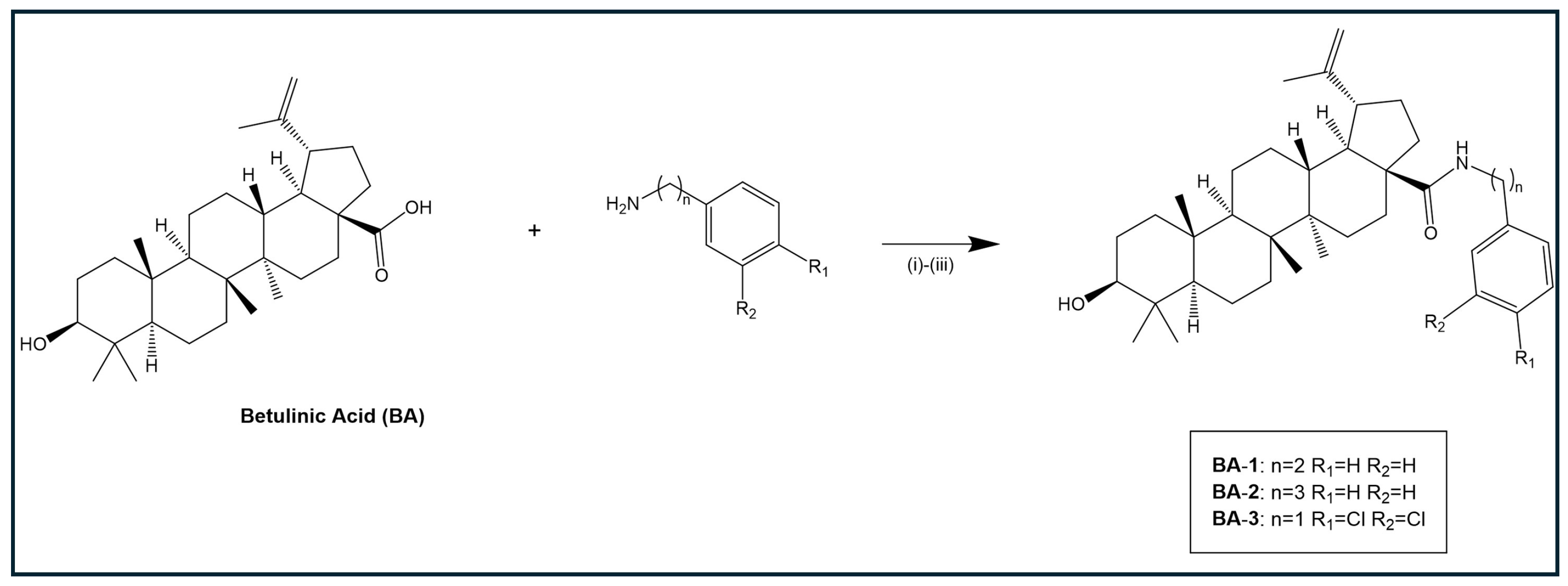
2. Results and Discussion
3. Materials and Methods
Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDC | 3-[(Ethylimino)methylidene]amino-N,N-dimethylpropan-1-amine |
| HOBT | Hydroxybenzotriazole |
| DIPEA | N,N-Diisopropylethylamine |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| MeOH | Methanol |
| ROS | Reactive oxygen species |
| BBB | Blood–brain barrier |
References
- Zhong, Y.; Liang, N.; Liu, Y.; Cheng, M.S. Recent progress on betulinic acid and its derivatives as antitumor agents: A mini review. Chin. J. Nat. Med. 2021, 19, 641–647. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.V.G.D.; Dos Reis, B.P.Z.C.; Soares, M.B.P. Anti–Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Xiang, H.M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non—Small Cell Lung Cancer: A Review. JAMA J. Am. Med. Assoc. 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Wendt, C.; Margolin, S. Identifying breast cancer susceptibility genes—A review of the genetic background in familial breast cancer. Acta Oncol. 2019, 58, 135–146. [Google Scholar] [PubMed]
- Manatakis, D.K.; Tasis, N.; Antonopoulou, M.I.; Kordelas, A.; Balalis, D.; Korkolis, D.P. Tseleni-Balafouta S, Colorectal cancer metastases to the thyroid gland—A systematic review: Colorectal cancer thyroid metastases. Hormones 2021, 20, 85–91. [Google Scholar]
- Sergi, C.M. Liver Cancer; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Dall’Era, M.A.; Cooperberg, M.R.; Chan, J.M.; Davies, B.J.; Albertsen, P.C.; Klotz, L.H.; Warlick, C.A.; Holmberg, L.; Bailey, D.E., Jr.; Wallace, M.E.; et al. Active surveillance for early-stage prostate cancer: Review of the current literature. Cancer 2008, 112, 1650–1659. [Google Scholar]
- Niculescu, A.G.; Grumezescu, A.M. Novel Tumor–Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Wimmer, Z. Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. Eur. J. Med. Chem. 2018, 145, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Anyanwu, M.; Leong, K.; Li, J.; Gianoncelli, A.; Coghi, P.; Ribaudo, G. 3-[(1H-Benzo[d][1,2,3]triazol-1-yl)oxy]propyl 9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylate. Molbank 2022, 2022, M1419. [Google Scholar] [CrossRef]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure—Activity Relationship Study of Betulinic Acid, A Novel and Selective TGR5 Agonist, and Its Synthetic Derivatives: Potential Impact in Diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef]
- Dangroo, N.A.; Singh, J.; Rath, S.K.; Gupta, N.; Qayum, A.; Singh, S.; Sangwan, P.L. A convergent synthesis of novel alkyne—Azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 2017, 123, 1–12. [Google Scholar] [CrossRef]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.P.; Csuk, R. Betulinic acid derived amides are highly cytotoxic, apoptotic and selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef] [PubMed]
- Eignerova, B.; Tichy, M.; Krasulova, J.; Kvasnica, M.; Rarova, L.; Christova, R.; Urban, M.; Bednarczyk-Cwynar, B.; Hajduch, M.; Sarek, J. Synthesis and antiproliferative properties of new hydrophilic esters of triterpenic acids. Eur. J. Med. Chem. 2017, 140, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, T.; Tian, X.; Han, N.; Liu, X.; Chen, H.; Qi, J.; Gao, F.; Li, W.; Wu, Q.; et al. Betulinic Acid-Nitrogen Heterocyclic Derivatives: Design, Synthesis, and Antitumor Evaluation In Vitro. Molecules 2020, 25, 948. [Google Scholar] [CrossRef]
- Guo, W.B.; Zhang, H.; Yan, W.Q.; Liu, Y.M.; Zhou, F.; Cai, D.S.; Zhang, W.X.; Huang, X.M.; Jia, X.H.; Chen, H.S.; et al. Design, synthesis, and biological evaluation of ligustrazine—Betulin amino-acid/dipeptide derivatives as anti-tumor agents. Eur. J. Med. Chem. 2020, 185, 111839. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kodr, D.; Stanková, J.; Rumlová, M.; Džubák, P.; Řehulka, J.; Zimmermann, T.; Křížová, I.; Gurská, S.; Hajdúch, M.; Drašar, P.B.; et al. Betulinic Acid Decorated with Polar Groups and Blue Emitting BODIPY Dye: Synthesis, Cytotoxicity, Cell-Cycle Analysis and Anti-HIV Profiling. Biomedicines 2021, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, Y.; Coluccini, C.; Coghi, P. ((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Acetoxy-5a,5b,8,8,11a- pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysen-3a-yl)methyl 2-Bromo-3-methylbenzoate. Molbank 2025, 2025, M1971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Xie, Y.; Qi, J.; Ren, Z.; Coluccini, C.; Coghi, P. Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization. Molbank 2025, 2025, M2072. https://doi.org/10.3390/M2072
Xu Q, Xie Y, Qi J, Ren Z, Coluccini C, Coghi P. Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization. Molbank. 2025; 2025(4):M2072. https://doi.org/10.3390/M2072
Chicago/Turabian StyleXu, Qinwei, Yuhan Xie, Jin Qi, Zimo Ren, Carmine Coluccini, and Paolo Coghi. 2025. "Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization" Molbank 2025, no. 4: M2072. https://doi.org/10.3390/M2072
APA StyleXu, Q., Xie, Y., Qi, J., Ren, Z., Coluccini, C., & Coghi, P. (2025). Development of New Amide Derivatives of Betulinic Acid: Synthetic Approaches and Structural Characterization. Molbank, 2025(4), M2072. https://doi.org/10.3390/M2072





