(5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione
Abstract
1. Introduction
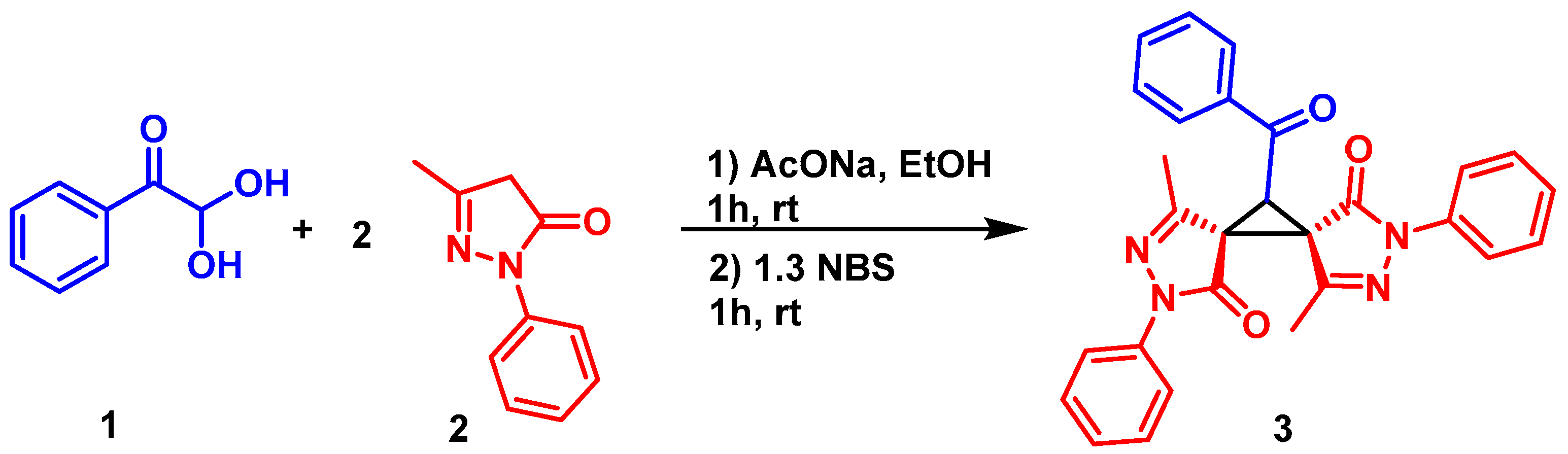
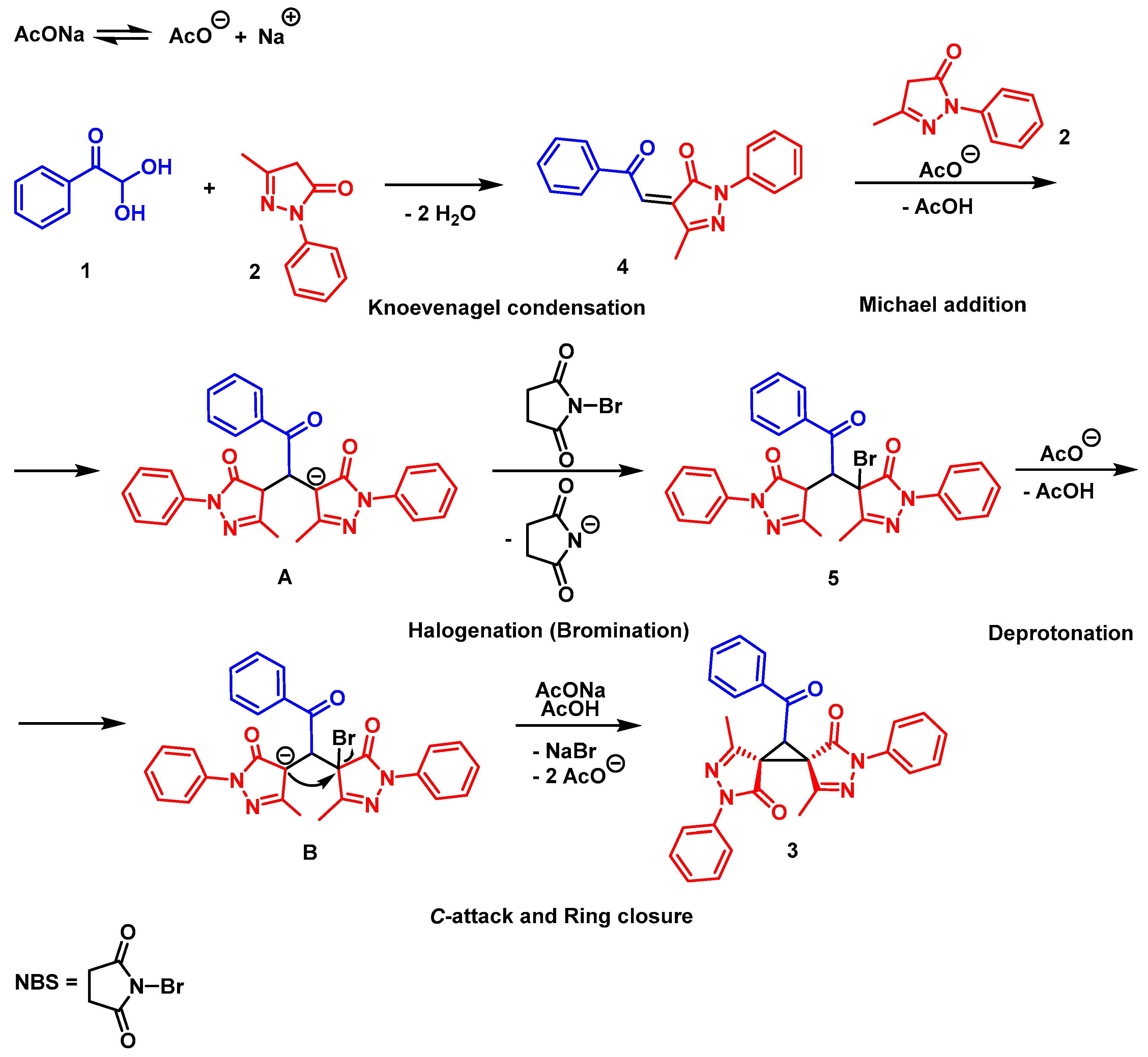
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of (5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.46.15]undeca-3,9-diene-1,7-dione (3)
3.3. (5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.4.615]undeca-3,9-diene-1,7-dione (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D. The roles of fused-cyclopropanes in medicinal chemistry: Insights from the past decade. Eur. J. Med. Chem. 2025, 297, 117951. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Uthumange, S.S.; Liew, A.J.H.; Chee, X.W.; Yeong, K.Y. Ringing medicinal chemistry: The importance of 3-membered rings in drug discovery. Bioorg. Med. Chem. 2024, 116, 117980. [Google Scholar] [CrossRef] [PubMed]
- Luckhurst, C.A.; Breccia, P.; Stott, A.J.; Aziz, O.; Birch, H.L.; Bürli, R.W.; Hughes, S.J.; Jarvis, R.E.; Lamers, M.; Leonard, P.M.; et al. Potent, Selective, and CNS-Penetrant Tetrasubstituted Cyclopropane Class IIa Histone Deacetylase (HDAC) Inhibitors. ACS Med. Chem. Lett. 2015, 7, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Basavaraja, D.; Siddalingeswar, V.D.; Athira, C.S.; Siby, A.; Sreelakshmi, V.; Ancy, A.; Sasidhar, B.S. Spiro-Heterocycles: Recent Advances in Biological Applications and Synthetic Strategies. Tetrahedron 2025, 173, 134468. [Google Scholar] [CrossRef]
- Varela, M.T.; Dias, G.G.; de Oliveira, L.F.N.; de Oliveira, R.G.; Aguiar, F.D.; Nogueira, J.P.; Cruz, L.R.; Dias, L.C. Spirocyclic compounds as innovative tools in drug discovery for medicinal chemists. Eur. J. Med. Chem. 2025, 287, 117368. [Google Scholar] [CrossRef]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic Motifs in Natural Products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef]
- Habibi, D.; Beiranvand, M. Nomenclature, Synthesis and Applications of Spirocyclic Compounds; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2023; ISBN 978-1-5275-3941-9. Available online: https://books.google.ru/books?id=ZdriEAAAQBAJ&printsec=frontcover&hl=ru#v=onepage&q&f=false (accessed on 13 October 2025).
- Kale, S.B.; Jori, P.K.; Thatikonda, T.; Gonnade, R.G.; Das, U. 1,6-Conjugate-addition-induced [2+1] annulation of para-quinone methides and pyrazolones: Synthesis of bis-spirocompounds with contiguous quaternary spiro-centers. Org. Lett. 2019, 21, 7736–7740. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shao, P.; Li, Y.; Wang, D.; Hou, D.; Qu, C.; Yan, X. Regioselective synthesis of spirobarbiturate-dihydrofurans and dihydrofuro [2,3-d]pyrimidines via one-pot cascade reaction of barbiturate-based olefins and ethyl acetoacetate. Tetrahedron 2021, 79, 131859. [Google Scholar] [CrossRef]
- Nakamura, A.; Rao, F.; Ukiya, K.; Matsunaga, R.; Ohiraa, S.; Maegawa, T. A concise synthesis of thioaurones via NBS-induced cyclization of MOM-protected 2′-mercaptochalcones. Org. Biomol. Chem. 2023, 21, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Ravikanth, M. NBS-Enabled rapid cyclization of 5,6-diaryl dipyrroethenes to access meso-tetraaryl porphycenes. Org. Lett. 2025, 27, 8679–8684. [Google Scholar] [CrossRef] [PubMed]
- Elinson, M.N.; Dorofeeva, E.O.; Vereshchagin, A.N.; Nasybullin, R.F.; Egorov, M.P. Electrocatalytic stereoselective transformation of aldehydes and two molecules of pyrazolin-5-one into (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes. Catal. Sci. Technol. 2015, 5, 2384–2387. [Google Scholar] [CrossRef]
- Grjol, B.; Jereb, M. Reactivity of substrates with multiple competitive reactive sites toward NBS under neat reaction conditions promoted by visible light. Chem. Pap. 2021, 75, 5235–5248. [Google Scholar] [CrossRef]
- Elinson, M.N.; Nasybullin, R.F.; Nikishin, G.I. Sodium acetate catalyzed tandem Knoevenagel–Michael multicomponent reaction of aldehydes, 2-pyrazolin-5-ones, and cyano-functionalized C–H acids: Facile and efficient way to 3-(5-hydroxypyrazol-4-yl)-3-aryl-propionitriles. Comptes Rendus Chim. 2013, 16, 789–794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elinson, M.N.; Kalashnikova, V.M.; Ryzhkova, Y.E.; Rakitin, O.A. (5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione. Molbank 2025, 2025, M2073. https://doi.org/10.3390/M2073
Elinson MN, Kalashnikova VM, Ryzhkova YE, Rakitin OA. (5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione. Molbank. 2025; 2025(4):M2073. https://doi.org/10.3390/M2073
Chicago/Turabian StyleElinson, Michail N., Varvara M. Kalashnikova, Yuliya E. Ryzhkova, and Oleg A. Rakitin. 2025. "(5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione" Molbank 2025, no. 4: M2073. https://doi.org/10.3390/M2073
APA StyleElinson, M. N., Kalashnikova, V. M., Ryzhkova, Y. E., & Rakitin, O. A. (2025). (5R*,6R*) 11-Benzoyl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro [4.0.46.15]undeca-3,9-diene-1,7-dione. Molbank, 2025(4), M2073. https://doi.org/10.3390/M2073








