Abstract
3-(4-hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one is a derivative of the well-known acid/base indicator, phenolphthalein. We report the synthesis and the molecular structure of the title compound as determined by single-crystal X-ray diffraction. 1H and 13C NMR spectroscopy, IR spectroscopy, and mass spectrometry data have been provided.
1. Introduction
Phenolphthalein (1), thymolphthalein (2) and cresolphthalein (3) are routinely used as pH indicators for titrimetric analysis (Figure 1) [1,2,3]. These compounds have also found wider applications in colour-changing paper products, paints, inks, coatings and adhesives [2].
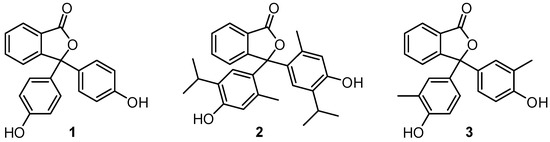
Figure 1.
Structures of phenolphthalein (1), thymolphthalein (2) and cresolphthalein (3).
Phenolphthalein and its derivatives are often prepared by heating phenol with phthalic anhydride in the presence of sulfuric acid, zinc chloride or aluminium chloride (Scheme 1) [1,2,3]. More recent work has identified milder conditions for the synthesis of phenolphthaleins, an important example being a recent report by Sabnis. The protocol described provides an efficient method that allows access to a range of substituted phenolphthaleins; the key feature is the use methanesulfonic acid as an alternative to concentrated sulfuric acid [1]. The protocol developed by Sabnis has subsequently been adapted further, and it has been found that addition of catalytic niobium(V) chloride can accelerate the rate of reaction of phenols with phthalic anhydride in methanesulfonic acid [4].
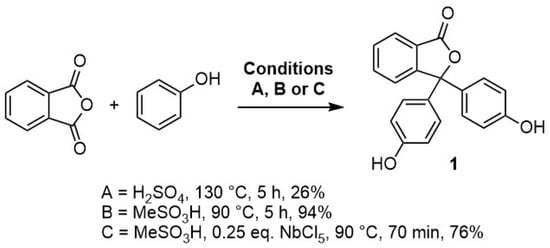
Scheme 1.
Synthetic routes to phenolphthalein using sulfuric acid, methanesulfonic acid, and methanesulfonic acid/niobium chloride.
Phenolphthaleins are halochromic and are particularly useful as pH indicators for basic solutions [1,2,5]. Under acidic solutions, phenolphthaleins exist as colourless phthalides; however, on treatment with a strong base, colourful triarylmethane dianions are formed (Scheme 2) [2,5]. Access to substituted phenolphthaleins allows a wider range of colours to be obtained in basic solutions. It has been shown that increasing the number of methyl groups on the phenolic substituents of phenolphthalein indicators leads to basic solutions becoming progressively bluer in colour (Scheme 2 and Table 1) [1,4].
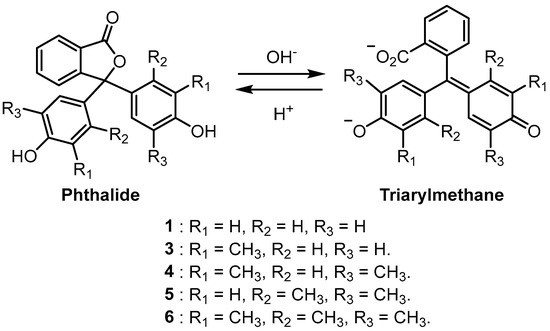
Scheme 2.
Phthalide and quinoidal dianion forms of phenolphthaleins.

Table 1.
Colours of phenolphthalein pH indicators in 0.1 M NaOH solution [1].
In this paper, we describe the results from an ongoing study of unsymmetrical analogues of phenolphthalein, where synthetic routes and physical properties of this class of compounds have been investigated. All the phenolphthalein examples highlighted in Table 1 are symmetrical, and our focus has been directed toward investigating the colour of basic solutions of phenolphthaleins where only one of the phenolic substituents has methyl groups attached. Unsymmetrical analogues of phenolphthaleins are known; however, these have received less attention in the literature than the more familiar symmetrical compounds [6,7,8]. Example methods for the preparation of unsymmetrical phenolphthaleins are outlined in Scheme 3. 2-(4-hydroxybenzoyl)benzoic acids are often used as starting materials; for example, the treatment of acid 7 with 2-methylphenol in the presence of zinc chloride affords compound 8 [7]. Similarly, acid 9 can be converted to compound 10 when it is reacted with thymol in concentrated sulfuric acid [8].
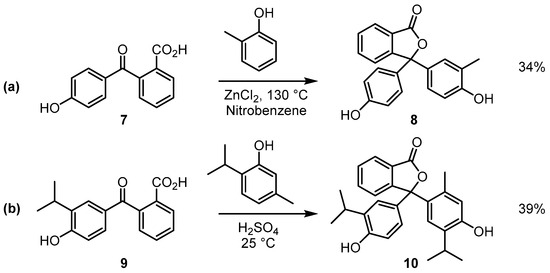
Scheme 3.
Reactions of 2-(4-hydroxybenzoyl)benzoic acids 7 (a) and 9 (b)with phenols under acidic conditions.
In this work, we report that a modified version of the conditions developed by Sabnis allows access to the previously unknown trimethylated phenolphthalein derivative 11 (Scheme 4a) [1]. It had previously been shown that the reaction of 2,3,6-trimethylphenol with phthalic anhydride in hot methanesulfonic acid afforded 2,3,6-trimethylphenolphthalein (6) (Scheme 4b) [1]. We have found that the reaction of 2-(4-hydroxybenzoyl)benzoic acid (7) (in place of phthalic anhydride) with 2,3,6-trimethylphenol in methanesulfonic acid leads to compound 11 in good yield [9]. NMR spectra and IR spectra of 11 have been acquired; furthermore, the molecular structure of compound 11 has been determined by single-crystal X-ray diffraction.
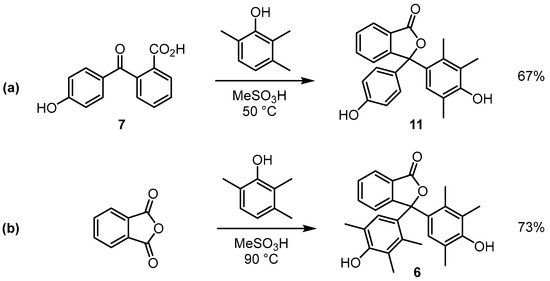
Scheme 4.
Reaction of 2,3,6-trimethylphenol with (a) 2-(4-hydroxybenzoyl)benzoic acid or (b) phthalic anhydride in acidic conditions.
2. Results and Discussion
2.1. Synthesis and Spectroscopy
Compound 11 was obtained in 67% yield from the reaction of 2,3,6-trimethylphenol with 2-(4-hydroxybenzoyl)benzoic acid (7) in warm methanesulfonic acid (50 °C) over a 1 h period. The reaction was quenched with water, and the resulting crude product was isolated by filtration. The product was then readily purified by recrystallisation from a mixture of methanol/water. A particularly notable difference was observed when compounds 6 and 11 were compared in basic solution. Previous work by Sabnis demonstrated that compound 6 forms a teal-coloured solution in dilute sodium hydroxide solution, and we have found that compound 11 results in a violet-coloured solution under the same conditions [1]. Figure 2 shows the results of adding a drop of 0.5% ethanolic solutions of compounds 6 or 11 to a 3 mL aliquot of 0.1 M sodium hydroxide. A set of UV-visible spectra of compounds 6 and 11 dissolved in dilute sodium hydroxide has been provided in the Supporting Information document (Supporting Information, Figure S1). The origin of the hypsochromic shift observed in the UV-vis absorption spectrum of 11 (compared to the spectrum of 6) is currently unclear and work on this will be disclosed in due course.

Figure 2.
Solutions of compounds 6 and 11 in 0.1 M sodium hydroxide.
A set of 1H and 13C NMR spectra has been provided in the supporting information document (Supporting information, Figures S2–S5). The 1H NMR spectrum of 11 is consistent with the structure. The 4-hydroxy-2,3,6-trimethylphenyl substituent has four associated singlets, three derived from the methyl groups (2.09, 2.05, and 1.93 ppm) and one from the sole aromatic hydrogen atom (6.68 ppm). There are two distinctive AA′BB′ signals (6.92 and 6.71 ppm) that are characteristic of the two hydrogen environments within the 4-hydroxyphenyl substituent. Furthermore, the hydrogen atoms from the isobenzofuranone moiety are also readily identified as the signals with the following chemical shifts: 7.90, 7.79, 7.62, and 7.51 ppm. The 13C NMR spectrum also provides diagnostic information, with the isobenzofuranone carbonyl carbon signal observed at 169.9 ppm and the quaternary spiro carbon atom linking the aromatic substituents to the isobenzofuranone ring observed at 93.3 ppm. The three methyl groups associated with the 4-hydroxy-2,3,6-trimethylphenyl substituent are observed at 18.4, 17.3, and 13.1 ppm. The ATR-IR spectrum of compound 11 has also been obtained (Supporting Information, Figure S6), showing a distinctive signal at 1714 cm−1 that is consistent with the carbonyl group within the isobenzofuranone moiety. In addition, a signal is observed at 3414 cm−1 due to the hydroxyl groups from each phenol substituent.
2.2. The Crystal Structure of 11
Crystals of 11 suitable for X-ray diffraction were obtained by slow crystallisation from methanol/water. The compound crystallises in the orthorhombic space group Fdd2 with one molecule in the asymmetric unit and connectivity consistent with the spectroscopic data (Figure 3). The spiro carbon (C3) of the phthalein ring shows distortion from ideal tetrahedral geometry with the angle constrained by the ring (O2–C3–C4) at 101.6(2)°, and other angles similarly larger. The adjacent O2–C3 bond is longer than is typical for γ-lactone structures (c.a. 1.46 Å) [10,11] at 1.494(3) Å, but is close to the reported distances of the equivalent bonds in the structure of 1 [11,12]. The phenol ring is twisted nearly perpendicular to the phthalein (74.09(12)°) while the trimethylphenol ring is more parallel (31.63(12)°), and the two phenol rings twisted nearly perfectly perpendicular (89.82(11)°) with respect to each other. This is in contrast to the conformation of the phenol rings in 1, where both rings in both independent units show a moderate twist (50–60°) with respect to the phthalide ring (Figure 3b).
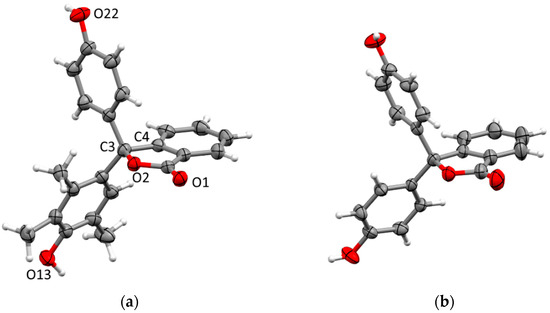
Figure 3.
(a) Crystal structure of 11 (selected numbering and ellipsoids drawn at 50% probability). (b) Crystal structure of 1 (literature data for comparison with 11 [12], ellipsoids drawn at 50% probability).
Both hydroxyl groups present in 11 form O–H···O hydrogen bonds to the carbonyl oxygen of adjacent molecules (H···O 1.82(3) and 1.91(3) Å, and O···O 2.731(3) and 2.835(3) for the phenol and trimethylphenol, respectively) which link adjacent molecules into chains along [0 1 −1] or [0 0 1] through the phenol or trimethylphenol hydroxyl, respectively (Figure 4 and Figure 5). Together, these chains form hydrogen-bonded sheets in the (1 0 0) plane. There are also weak non-classical C–H···O hydrogen bonds observed (H···O 2.533(2) and 2.599(3) Å, C···O 3.376(4) and 3.357(4) Å) from hydrogens of the phthalide ring to hydroxyl oxygens of adjacent molecules, forming weakly interacting sheets in the (0 1 0) plane and linking the conventionally hydrogen-bonded sheets into the overall 3D structure (Supporting Information, Figure S8).
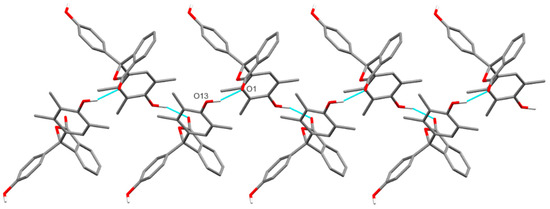
Figure 4.
Hydrogen-bonded chains of 11 along [0 0 1] resulting from trimethylphenol hydroxyl to carbonyl O–H···O hydrogen bonds (blue dashed lines). (Carbon-bound hydrogen atoms omitted for clarity).
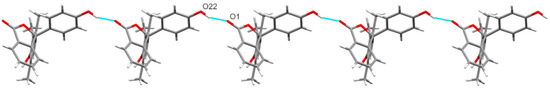
Figure 5.
Hydrogen-bonded chains of 11 along [0 1 −1] resulting from phenol hydroxyl to carbonyl O–H···O hydrogen bonds (blue dashed lines).
In summary, the first synthesis of 3-(4-hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one (11) has been reported. The title compound was prepared by heating a mixture of 2-(4-hydroxybenzoyl)benzoic acid (7) and 2,3,6-trimethylphenol in methanesulfonic acid. NMR and IR spectroscopy data have been acquired; furthermore, the molecular structure of the title compound has been determined by single-crystal X-ray diffraction. This study presents a simple synthetic route to a previously unknown acid/base indicator related to phenolphthalein.
3. Experimental Section
2-(4-Hydroxybenzoyl)-benzoic acid (7) was prepared from phenolphthalein (1) by a literature method reported by Hubacher [9]. Melting points were recorded on a Stuart SMP3 melting point apparatus (tolerance ±1.5 °C at 300 °C) (Mettler Toledo Ltd., Leicester, UK). The melting point apparatus was calibrated using samples of phenolphthalein (m.p. 261–263 °C). IR spectra were recorded on a Nicolet Summit FTIR instrument with an Everest diamond ATR accessory (Fisher Scientific, Loughborough, UK). NMR spectra were obtained for 1H at 400.3 MHz and for 13C at 100.67 MHz using a Bruker AVIII_HD 400 instrument (Bruker UK Ltd., Coventry, UK). Spectra were run at 25 °C in DMSO-d6. Chemical shifts are reported in ppm to high frequency of the reference and coupling constants J are reported in Hz. The HRMS data were acquired from the University of St Andrews Mass Spectrometry Service.
3-(4-Hydroxy-2,3,6-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one (11)
2-(4-Hydroxybenzoyl)-benzoic acid (0.4 g, 1.64 mmol) and 2,3,6-trimethylphenol (0.23 g, 1.64 mmol) and 1.0 g of methanesulfonic acid were stirred magnetically and heated to 50 °C for 1 h. The reaction was quenched with water (20 mL) and the resulting mixture was heated in a water bath (100 °C) for 10 min. On cooling, the crude product was filtered off under suction and recrystallised from a mixture of methanol and water (0.40 g, 1.11 mmol, 67%). m.p. 234–236 °C; IR (ATR) 3414 (OH), 3024, 2977, 2921 (CH), 1714 (C=O), 1595, 1511, 1471, 1295, 1136, 937, 925, 829 cm−1; 1H NMR (400 MHz, DMSO-d6): 9.52 (1H, s, OH), 8.42 (1H, s, OH), 7.90 (1H, d, J = 7.6 Hz, ArH), 7.79 (1H, td, J = 7.5, 1.0 Hz, ArH), 7.62 (1H, t, J = 7.6, ArH), 7.51 (1H, d, J = 7.5 Hz, ArH), 6.92 (2H, d, J = 8.6 Hz, ArH), 6.71 (2H, d, J = 8.6, Hz, ArH), 6.68 (1H, s, ArH), 2.09 (3H, s, CH3), 2.05 (3H, s, CH3), 1.93 (3H, s, CH3);. 13C NMR (100 MHz, DMSO-d6): 169.9 (C=O), 157.3 (ArCq), 153.9 (ArCq), 153.8 (ArCq), 133.3 (ArCq), 134.8 (ArCH), 135.8 (ArCq), 129.8 (ArCH), 128.7 (ArCq), 127.9 (ArCH), 126.4 (ArCq), 126.3 (ArCH), 126.0 (ArCH), 125.9 (ArCH), 124.5 (ArCq), 120.4 (ArCq), 116.1 (ArCH), 93.3 (Cq), 18.4 (CH3), 17.3 (CH3), 13.1 (CH3); HRMS (ESI+) m/z (%) Calcd. for C23H20O4Na 383.1251, found 383.1254 [M + Na] (100).
Colourless X-ray quality crystals of 11 were obtained by slow crystallisation of samples from methanol/water.
X-ray Structure Determination of 11
X-ray diffraction data for 11 were collected at 173 K using a Rigaku SCXmini CCD diffractometer with a SHINE monochromator [Mo Kα radiation (λ = 0.71073 Å)] (Rigaku USA, The Woodlands, TX, USA). Data were collected (using a calculated strategy) and processed (including correction for Lorentz, polarisation and absorption) using CrysAlisPro (v1.171.43.109a & 129a) [13]. The structure was solved by dual-space methods (SHELXT) and refined by full-matrix least-squares against F2 (SHELXL-2019/3) [14,15]. Non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using a riding model except for the hydrogen atoms on O13 and O22 which were located from the difference Fourier map and refined isotropically, subject to a distance restraint. All calculations were performed using the Olex2 interface [16].
Selected crystallographic data: C23H20O4, M = 360.39, orthorhombic, a = 45.4638(11), b = 18.1317(5), c = 9.1832(2) Å, U = 7570.0(3) Å3, T = 173 K, space group Fdd2 (no. 43), Z = 16, 53,406 reflections measured, 6749 unique (Rint = 0.0624), which were used in all calculations. The final R1 [I > 2σ(I)] was 0.0574 and wR2 (all data) was 0.1603. CCDC 2,487,059 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Supplementary Materials
The following are available online. Figure S1: UV-vis spectra of the dianion forms of compounds 6 and 11; Figure S2: 400 MHz 1H-NMR spectrum of 11; Figure S3: 1H-1H COSY NMR spectrum of 11; Figure S4: 100 MHz 13C DEPTQ NMR spectrum of 11; Figure S5: 1H-13C HSQC NMR spectrum of 11; Figure S6: ATR-IR spectrum of 11; Figure S7: ESI-mass spectrum of 11; CIF file for compound 11; Table S1: Hydrogen bond metrics (Å or °) of 1 and 11; Figure S8: Packing of 11 into sheets across (1 0 0) from combination of hydrogen-bonded chains; Figure S9: Hydrogen-bonded chains of 11 along [0 0 1]; Figure S10: Hydrogen-bonded chains of 11 along [0 1 −1]; Figure S11: Hydrogen-bonded chains of each independent molecule of 1 along either [0 0 1] or [0 1 −1].
Author Contributions
Synthetic steps, crystallisation trials, and preliminary analysis were conducted by N.V., I.A.S. and I.L.J.P.; A.P.M. and D.B.C. collected the X-ray data and solved the structure; B.A.C., D.B.C., A.P.M., R.J.P. and I.A.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2487059 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Acknowledgments
The authors express gratitude to the University of St Andrews School of Chemistry for use of laboratory facilities and provision of materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sabnis, R.W. A facile synthesis of phthalein dyes. Tetrahedron Lett. 2009, 50, 6261–6263. [Google Scholar] [CrossRef]
- Sabnis, R.W. Developments in the chemistry and applications of phthalein dyes. Part 1: Industrial applications. Color. Technol. 2018, 134, 187–205. [Google Scholar] [CrossRef]
- Baeyer, A. Ueber die Phenolfarbstoffe. Ber. Dtsch. Chem. Ges. 1871, 4, 658–665. [Google Scholar] [CrossRef]
- Moreno, V.F.; dos Santos, G.C.; da Costa, G.M.G.; Gomes, M.H.A.; da Silva-Filho, L.C. NbCl5 Promoted the efficient synthesis of phthalein derivatives: Optical characterization and solvatochromic effect. J. Heterocycl. Chem. 2019, 56, 2811–2821. [Google Scholar] [CrossRef]
- Wittke, G. Reactions of Phenolphthalein at Various pH Values. J. Chem. Educ. 1983, 60, 239–240. [Google Scholar] [CrossRef]
- Lund, H. CCIV.—The constitution of phenolphthalein. Part 1. Preparation of some compounds of the phthalein type. J. Chem. Soc. 1928, 1569–1575. [Google Scholar] [CrossRef]
- Nekhoroshev, S.V.; Nekhoroshev, V.P.; Poleshchuk, O.K.; Yarkova, A.G.; Nekhorosheva, A.V.; Gasparyan, A.K. New chemical markers based on phthaleins. Russ. J. Appl. Chem. 2015, 88, 711–718. [Google Scholar] [CrossRef]
- Rumiński, J.K.; Przewoska, K.D. Synthesis and Reactivity of 2-Aroylbenzoic Acids, II. 2-(4-Hydroxy-3-isopropylbenzoyl)benzoic Acid. Chem. Ber. 1982, 115, 3436–3443. [Google Scholar] [CrossRef]
- Hubacher, M.H. The Preparation of 2-(Hydroxybenzoyl)-benzoic Acid. J. Am. Chem. Soc. 1946, 68, 718–719. [Google Scholar] [CrossRef]
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Typical interatomic distances: Organic compounds. In International Tables for Crystallography, 3rd ed.; Allen, F.H., Watson, D.G., Brammer, L., Orpen, A.G., Taylor, R., Eds.; John Wiley & Sons: New York, NY, USA, 2006; Volume 3, pp. 790–811. [Google Scholar]
- Sugira, H.; Kato, T.; Senda, H.; Kunimoto, K.K.; Kuwae, A.; Hanai, K. Crystal Structure of Phenolphthalein. Anal. Sci. 1999, 15, 611–612. [Google Scholar] [CrossRef]
- Fitzgerald, L.J.; Gerkin, R.E. Phenolphthalein and 3′,3′′-Dinitrophenolphthalein. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 535–539. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, v1.171.43.109a & 129a; Rigaku Oxford Diffraction, Rigaku Corporation: Tokyo, Japan, 2024.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).