Synthesis of (Camphor-3-yl)acetic Acid-Derived Pyrazoles
Abstract
1. Introduction
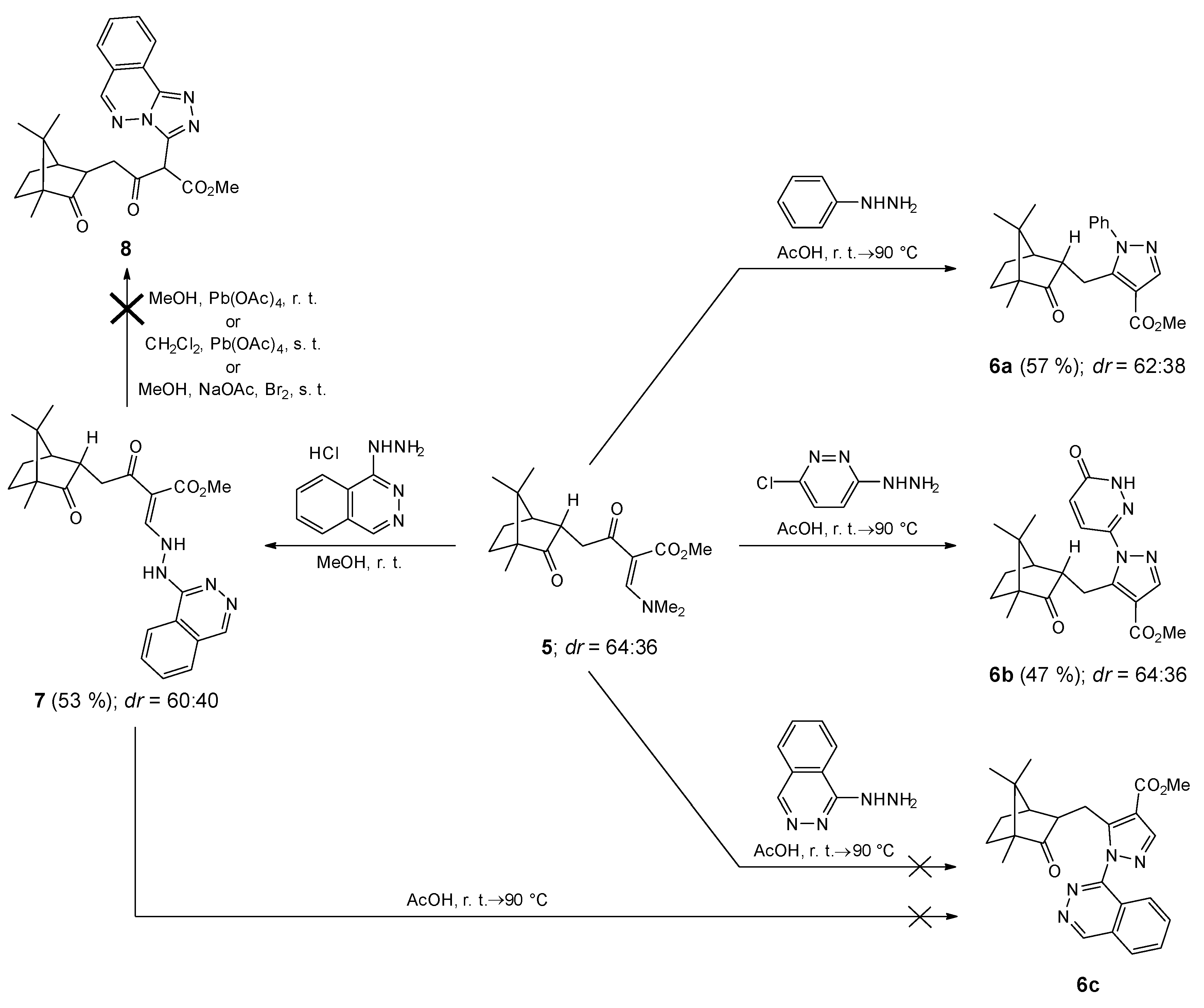
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2-((1R,2S,4S)-4,7,7-Trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)acetic Acid (3) and 2-((1R,2R,4S)-4,7,7-Trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)acetic Acid (3′)
3.2. Synthesis of Methyl 3-Oxo-4-((1R,2S,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (4) and Methyl 3-Oxo-4-((1R,2R,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (4′)
3.3. Synthesis of Methyl (Z)-2-((Dimethylamino)methylene)-3-oxo-4-((1R,2S,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (5) and Methyl (Z)-2-((Dimethylamino)methylene)-3-oxo-4-((1R,2R,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (5′)
3.4. Synthesis of Methyl 1-Phenyl-5-(((1R,2S,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)methyl)-1H-pyrazole-4-carboxylate (6a) and Methyl 1-Phenyl-5-(((1R,2R,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)methyl)-1H-pyrazole-4-carboxylate (6a′)
3.5. Synthesis of Methyl 1-(6-Oxo-1,6-dihydropyridazin-3-yl)-5-(((1R,2S,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)methyl)-1H-pyrazole-4-carboxylate (6b) and Methyl 1-(6-Oxo-1,6-dihydropyridazin-3-yl)-5-(((1R,2R,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)methyl)-1H-pyrazole-4-carboxylate (6b’)
3.6. Synthesis of Methyl (Z)-3-Oxo-2-((2-(phthalazin-1-yl)hydrazineyl)methylene)-4-((1R,2S,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (7) and Methyl (Z)-3-Oxo-2-((2-(phthalazin-1-yl)hydrazineyl)methylene)-4-((1R,2R,4S)-4,7,7-trimethyl-3-oxobicyclo [2.2.1]heptan-2-yl)butanoate (7′)
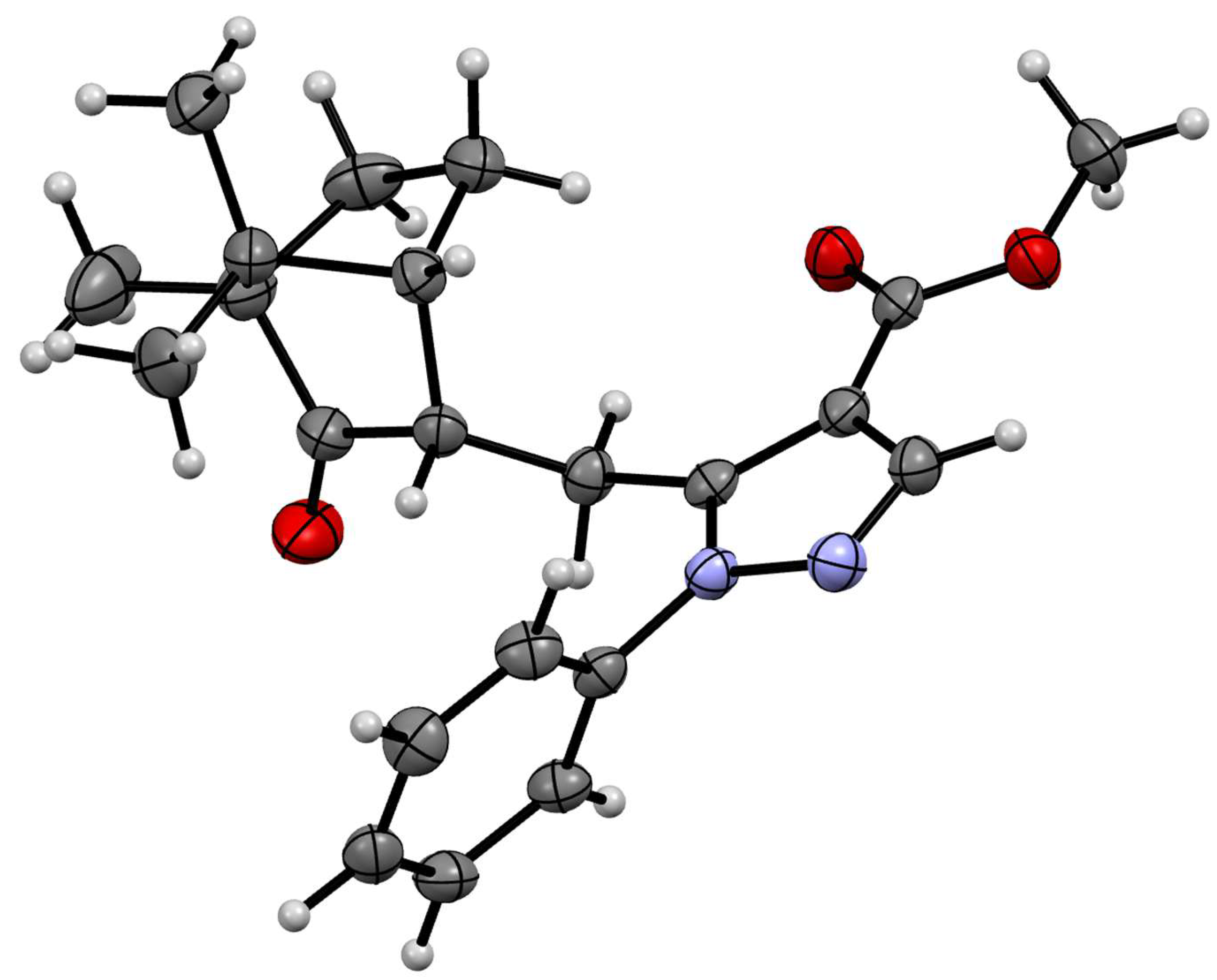
3.7. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Menezes, R.A.; Bhat, K.S. Synthetic aspects, structural insights and pharmacological potential of pyrazole derivatives: An overview. Discov. Appl. Sci. 2025, 7, 137. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Abdelgawad, M.A.; Elkanzi, N.A.A.; Bakr, R.B. Review of the recent advances of pyrazole derivatives as selective COX-2 inhibitors for treating inflammation. Mol. Divers. 2025, 29, 1789–1820. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Zahoor, A.F.; Ahmad, S. Pyrazole bearing molecules as bioactive scaffolds: A review. J. Chil. Chem. Soc. 2020, 65, 4746–4753. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef]
- Ram, K.; Raksha, S.; Dinesh Kumar, S. Pyrazole: A Privileged Scaffold of Medicinal Chemistry: A Comprehensive Review. Curr. Top. Med. Chem. 2023, 23, 2097–2115. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Kachhadiya, R.; Shah, D.; Prajapati, A.; Patel, A. Green Synthetic Strategies for Pyrazole Derivatives: A Comprehensive Review. Arch. Pharm. 2025, 358, e70055. [Google Scholar] [CrossRef]
- Fustero, S.; Simón-Fuentes, A.; Sanz-Cervera, J.F. Recent Advances in the Synthesis of Pyrazoles. A Review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Rostami, H.; Shiri, L.; Khani, Z. Recent advances in the synthesis of pyrazole scaffolds via nanoparticles: A review. Tetrahedron 2022, 110, 132688. [Google Scholar] [CrossRef]
- Ameziane El Hassani, I.; Rouzi, K.; Assila, H.; Karrouchi, K.; Ansar, M. Recent Advances in the Synthesis of Pyrazole Derivatives: A Review. Reactions 2023, 4, 478–504. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef] [PubMed]
- Money, T. Remote functionalization of camphor: Application to natural product synthesis. In Organic Synthesis: Theory and Applications; Hudlicky, T., Ed.; JAI Press: Stamford, CT, USA, 1996; Volume 3, pp. 1–83. [Google Scholar]
- Money, T. Camphor: A chiral starting material in natural product synthesis. Nat. Prod. Rep. 1985, 2, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Noe, C.R. Chirale Lactole, I. Die 2,3,3a,4,5,6,7a-Octahydro-7,8,8-trimethyl-4,7-methanobenzofuran-2-yl-Schutzgruppe. Chem. Ber. 1982, 115, 1576–1590. [Google Scholar] [CrossRef]
- Halterman, R.L.; Vollhardt, K.P.C. Synthesis and asymmetric reactivity of enantiomerically pure cyclopentadienylmetal complexes derived from the chiral pool. Organometallics 1988, 7, 883–892. [Google Scholar] [CrossRef]
- Knollmüller, M.; Gärtner, P.; Ferencic, M.; Noe, C.R.; Mereiter, K. An Improved Method for the endo-Fusion of Five-Membered Ring Lactones to the Bornane Ring System. Eur. J. Org. Chem. 1998, 1998, 2507–2511. [Google Scholar] [CrossRef]
- Noe, C.R.; Knollmüller, M.; Wagner, E. Ein einfaches Verfahren zur Herstellung anellierter Thiophene. Monatsh. Chem. 1986, 117, 621–629. [Google Scholar] [CrossRef]
- Noe, C.R.; Knollmüller, M.; Wagner, E.; Völlenkle, H. Kohlenhydrat-Modelle, I. Kinetische und thermodynamische Effekte bei Acetalisierungsreaktionen enantiomerenreiner Thiolactole. Chem. Ber. 1985, 118, 3299–3310. [Google Scholar] [CrossRef]
- Grošelj, U.; Bevk, D.; Jakše, R.; Meden, A.; Stanovnik, B.; Svete, J. Synthesis of (1R,4E,5S)-4-[[(E)-(azinyl)diazenyl]methylidene]-1,8,8-trimethyl-2-oxabicyclo[3.2.1]octan-3-ones and (1R,4R,5R)-4-([1,2,4]triazolo[4,3-x]azin-3-yl)-1,8,8-trimethyl-2-oxabicyclo[3.2.1]octan-3-ones. Tetrahedron Asymmetry 2005, 16, 2927–2945. [Google Scholar] [CrossRef]
- Grošelj, U.; Bevk, D.; Jakše, R.; Meden, A.; Stanovnik, B.; Svete, J. Reductions of (1R,3R,4R)-3-([1,2,4]triazolo[4,3-x]azin-3-yl)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ones and their analogues. Tetrahedron Asymmetry 2006, 17, 79–91. [Google Scholar] [CrossRef]
- Grošelj, U.; Rečnik, S.; Svete, J.; Meden, A.; Stanovnik, B. Stereoselective synthesis of (1R,3R,4R)-3-(1,2,4-triazolo[4,3-x]azin-3-yl)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ones. Tetrahedron Asymmetry 2002, 13, 821–833. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies UK Ltd.: Yarnton, UK, 2011.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cristallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciber, L.; Brodnik, H.; Petek, N.; Požgan, F.; Svete, J.; Štefane, B.; Grošelj, U. Synthesis of (Camphor-3-yl)acetic Acid-Derived Pyrazoles. Molbank 2025, 2025, M2058. https://doi.org/10.3390/M2058
Ciber L, Brodnik H, Petek N, Požgan F, Svete J, Štefane B, Grošelj U. Synthesis of (Camphor-3-yl)acetic Acid-Derived Pyrazoles. Molbank. 2025; 2025(3):M2058. https://doi.org/10.3390/M2058
Chicago/Turabian StyleCiber, Luka, Helena Brodnik, Nejc Petek, Franc Požgan, Jurij Svete, Bogdan Štefane, and Uroš Grošelj. 2025. "Synthesis of (Camphor-3-yl)acetic Acid-Derived Pyrazoles" Molbank 2025, no. 3: M2058. https://doi.org/10.3390/M2058
APA StyleCiber, L., Brodnik, H., Petek, N., Požgan, F., Svete, J., Štefane, B., & Grošelj, U. (2025). Synthesis of (Camphor-3-yl)acetic Acid-Derived Pyrazoles. Molbank, 2025(3), M2058. https://doi.org/10.3390/M2058








