1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate
Abstract
1. Introduction
2. Results and Discussion
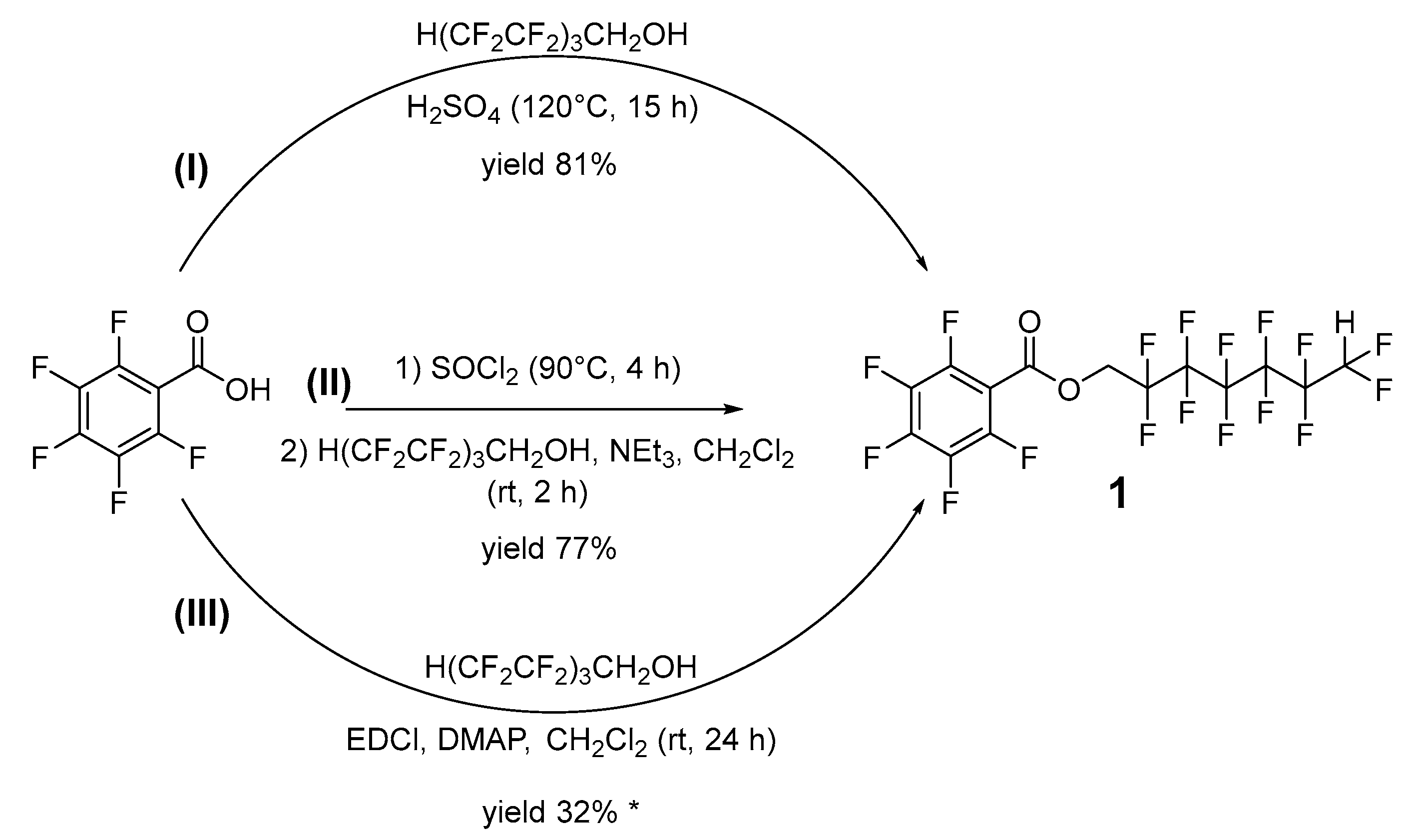
2.1. Acid-Catalyzed Esterification (Approach I)
2.2. Acyl Chloride Alcoholysis (Approach II)
2.3. Steglich-Type Esterification (Approach III)
3. Materials and Methods
3.1. General Information
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, J.I.; Grotjahn, S.; Senaweera, S.; Koenig, B.; Weaver Iii, J.D. Defluorodearomatization: A Photocatalytic Birch-Like Reduction That Enables C–C Bond Formation and Provides Access to Unnatural Cannabinoids. J. Org. Chem. 2021, 86, 7928–7945. [Google Scholar] [CrossRef] [PubMed]
- Weidner, V.L.; Barger, C.J.; Delferro, M.; Lohr, T.L.; Marks, T.J. Rapid, Mild, and Selective Ketone and Aldehyde Hydroboration/Reduction Mediated by a Simple Lanthanide Catalyst. ACS Catal. 2017, 7, 1244–1247. [Google Scholar] [CrossRef]
- Wang, S.; Golokhvastova, D.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; Gatilov, Y.V. Reduction of Perfluorinated Benzocycloalkenones and Other Polyfluoroaryl Ketones to Alcohols with LiBH4. Russ. J. Org. Chem. 2022, 58, 780–790. [Google Scholar] [CrossRef]
- Kalashnikov, S.B.; Vinogradov, A.S.; Yu. Bagryanskaya, I.; Mezhenkova, T.V. Synthesis of 5-methyl-4-polyfluoroaryl-1,3-thiazol-2-amines. Mendeleev Commun. 2024, 34, 277–278. [Google Scholar] [CrossRef]
- Kaletina, P.M.; Vinogradov, A.S.; Shaparenko, N.O.; Parkhomenko, D.A.; Shundrina, I.K.; Mezhenkova, T.V.; Bagryanskaya, E.G. Pentafluorophenyl vinyl ketone: Synthesis, radical polymerization, copolymerization, and polymer degradation under ultra-violet irradiation. J. Polym. Sci. 2023, 61, 2462–2474. [Google Scholar] [CrossRef]
- Vinogradov, A.S.; Platonov, V.E. Reactions of polyfluoroaromatic zinc compounds with oxalyl chloride. The synthesis of 1,2-bis(polyfluoroaryl)ethane-1,2-diones. Russ. Chem. Bull. 2023, 72, 2439–2445. [Google Scholar] [CrossRef]
- Stoesser, J.; Engelage, E.; Huber, S.M. Synthesizing Highly Fluorinated Oligophenyls via Negishi Coupling of Fluoroarylzinc Pivalates. Synthesis 2021, 54, 711–722. [Google Scholar] [CrossRef]
- Yi, X.; Mao, R.; Lavrencic, L.; Hu, X. Photocatalytic Decarboxylative Coupling of Aliphatic N-hydroxyphthalimide Esters with Polyfluoroaryl Nucleophiles. Angew. Chem. Int. Ed. 2021, 60, 23557–23563. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Zhang, Y.; Gao, Q.; Neumann, K.; Dong, H.; Porter, H.; Potter, M.; Ren, H.; Argyle, D.; Bradley, M. Switching on prodrugs using radiotherapy. Nat. Chem. 2021, 13, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Keana, J.F.W. Rapid and Selective Reduction of Functionalized Fluoroaryl Azides to the Corresponding Anilines with Stannous Chloride Dihydrate. Synth. Commun. 1993, 23, 357–360. [Google Scholar] [CrossRef]
- Xie, S.; Fukumoto, R.; Ramström, O.; Yan, M. Anilide Formation from Thioacids and Perfluoroaryl Azides. J. Org. Chem. 2015, 80, 4392–4397. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.M.; Hamilton, M.; Keen, B.; Despain, M.; Day, J.; Weaver, J.D. A Selective Single Step Amidation of Polyfluoroarenes. J. Fluor. Chem. 2021, 248, 109821. [Google Scholar] [CrossRef] [PubMed]
- Laev, S.S.; Evtefeev, V.U.; Shteingarts, V.D. A new approach to polyfluoroaromatic amines with an unsubstituted position ortho to the amino group. J. Fluor. Chem. 2001, 110, 43–46. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Ramström, O.; Yan, M. Base-catalyzed synthesis of aryl amides from aryl azides and aldehydes. Chem. Sci. 2016, 7, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, C.; Tang, X.; Yin, X.; Zhang, B. Highly selective synthesis of 1-polyfluoroaryl-1,2,3-triazoles via a one-pot three-component reaction. Tetrahedron Lett. 2014, 55, 5033–5037. [Google Scholar] [CrossRef]
- Riddhidev, B.; Endri, K.; Sabitri, L.; Kotsull, L.N.; Nishanth, K.; Dragan, I.; Mary Kay, H.P.; James, S.; William, T.; Viranga, T.L.M. Rational design of metabolically stable HDAC inhibitors: An overhaul of trifluoromethyl ketones. Eur. J. Med. Chem. 2022, 244, 114807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, T.; Zhu, S.; Xu, L. Solvent controlled regioselective reaction of 1,2,3-benzotriazole (BtH) with pentafluorobenzene derivatives. Tetrahedron 2011, 67, 910–914. [Google Scholar] [CrossRef]
- Chambers, R. Fluorine in Organic Chemistry; Wiley-Blackwell: Oxford, UK, 2004; pp. 296–364. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Completely Revised and Enlarged Edition; Wiley-VCH: Berlin, Germany, 2013; pp. 245–298. [Google Scholar]
- Wang, K.; Rahman, M.S.; Szilvási, T.; Gold, J.I.; Bao, N.; Yu, H.; Abbott, N.L.; Mavrikakis, M.; Twieg, R.J. Influence of multifluorophenyloxy terminus on the mesomorphism of the alkoxy and alkyl cyanobiphenyl compounds in search of new ambient nematic liquid crystals and mixtures. Liq. Cryst. 2021, 48, 672–688. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshida, K.; Kataoka, M.; Hara, M.; Konno, T. Donor-π-Acceptor-Type Fluorinated Tolane Containing a Semifluoroalkoxy Chain as a Condensed-Phase Luminophore. Molecules 2023, 28, 2764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.K.; Sharma, C. Esterification of fluorinated aromatic carboxylic acids with methanol by using UiO-66-NH2 as a heterogeneous catalyst and process optimization by the Taguchi method. RSC Adv. 2023, 13, 16712–16723. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kascheeva, S.S.; Lastovka, A.V.; Vinogradov, A.S.; Parkhomenko, D.A. 1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate. Molbank 2025, 2025, M2053. https://doi.org/10.3390/M2053
Kascheeva SS, Lastovka AV, Vinogradov AS, Parkhomenko DA. 1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate. Molbank. 2025; 2025(3):M2053. https://doi.org/10.3390/M2053
Chicago/Turabian StyleKascheeva, Sofia S., Anastasiya V. Lastovka, Andrey S. Vinogradov, and Dmitriy A. Parkhomenko. 2025. "1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate" Molbank 2025, no. 3: M2053. https://doi.org/10.3390/M2053
APA StyleKascheeva, S. S., Lastovka, A. V., Vinogradov, A. S., & Parkhomenko, D. A. (2025). 1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate. Molbank, 2025(3), M2053. https://doi.org/10.3390/M2053





