Methyl α-d-Tagatopyranoside
Abstract
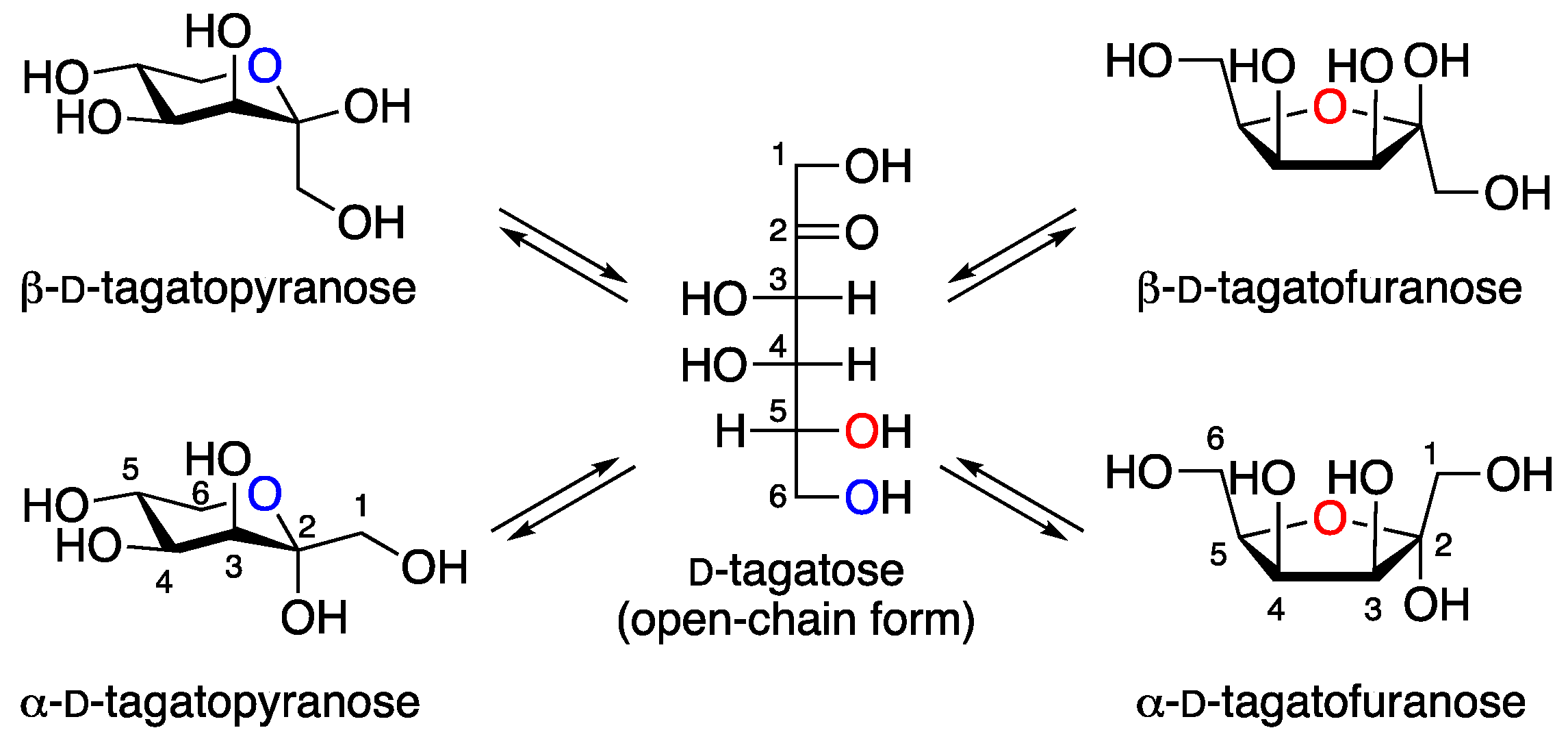
1. Introduction
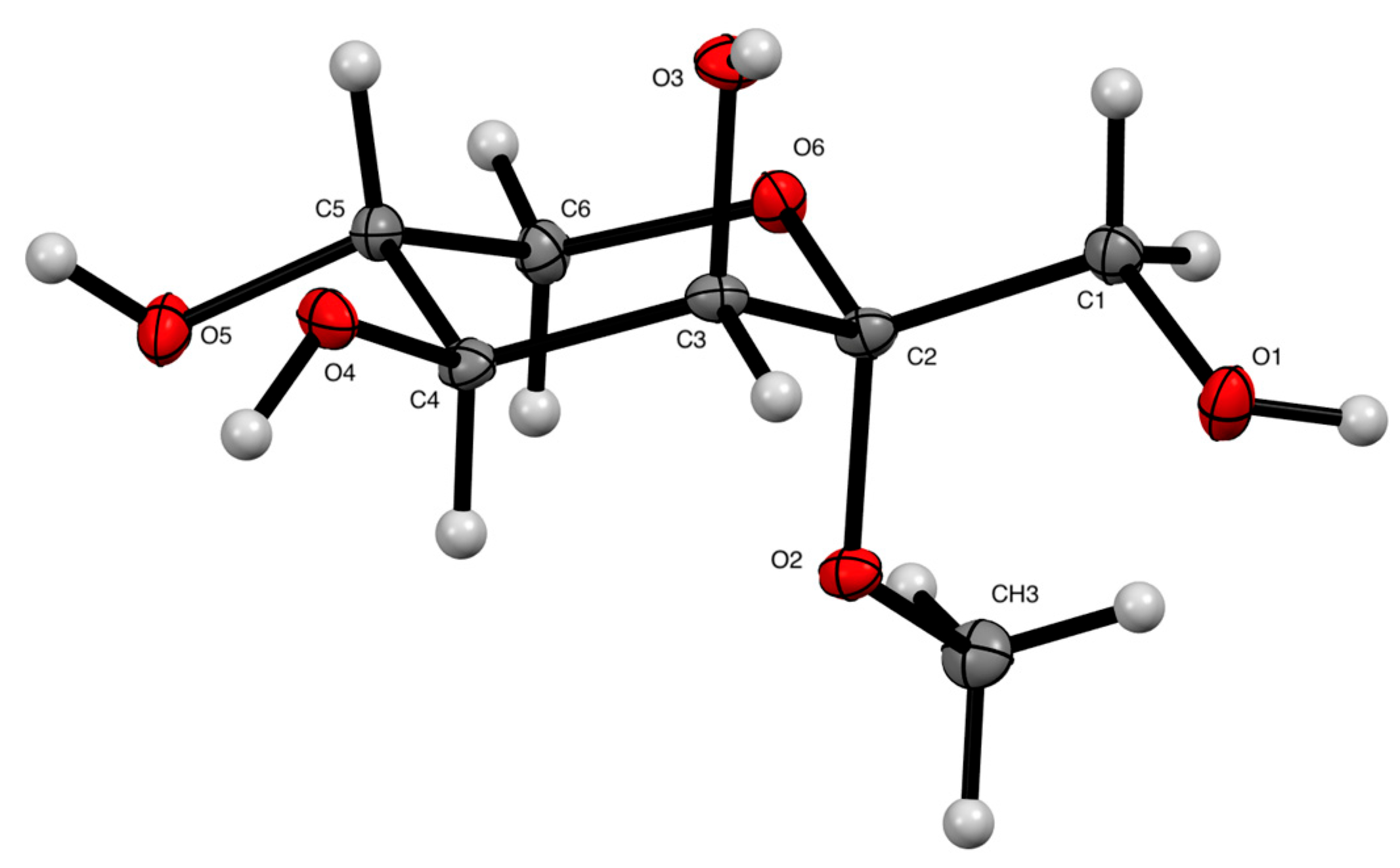
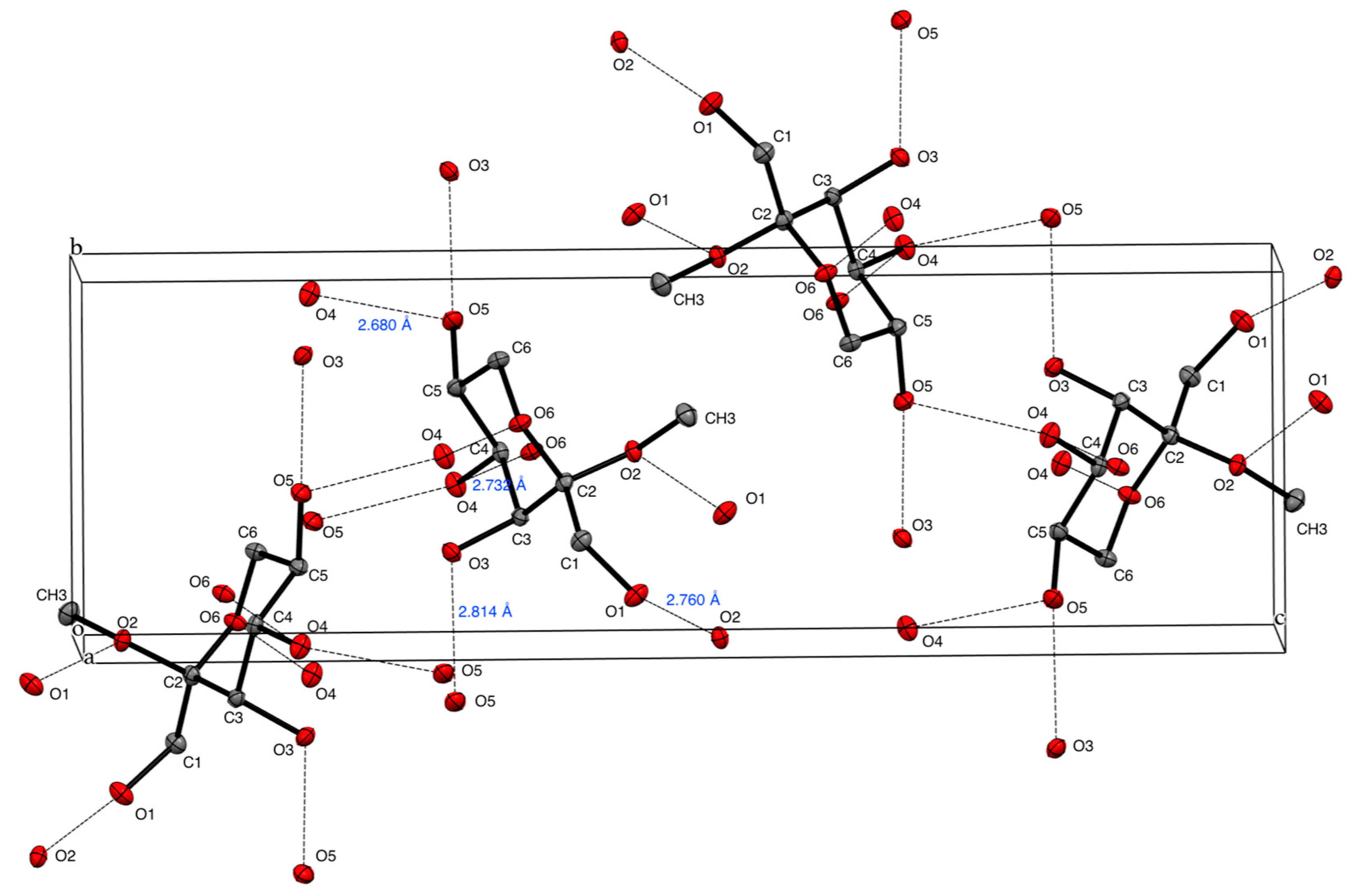
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure and Method
3.2. Methyl α-d-Tagatopyranoside (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COSY | Homonuclear Correlation Spectroscopy |
| HSQC | Heteronuclear Single Quantum Coherence |
| HMBC | Heteronuclear Multiple Bond Coherence |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
References
- Granstrom, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K. d-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef]
- Hayashi, N.; Yamada, T.; Takamine, S.; Iida, T.; Okuma, K.; Tokuda, M. Weight reducing effect and safety evaluation of rare sugar syrup by a randomized double-blind, parallel-group study in human. J. Funct. Foods 2014, 11, 152–159. [Google Scholar] [CrossRef]
- Izumori, K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol. 2006, 124, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef]
- Livesey, G.; Brown, J.C. d-Tagatose Is a Bulk Sweetener with Zero Energy Determined in Rats. J. Nutr. 1996, 126, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the New GRAS Sweetener and Health Product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V.; Zehner, L.R.; Saunders, J.P.; Beadle, J.R. Sugar substitutes: Their energy values, bulk characteristics, and potential health benefits. Am. J. Clin. Nutr. 1995, 62, 1161S–1168S. [Google Scholar] [CrossRef]
- Watkin, D.J.; Glawar, A.F.G.; Soengas, R.; Skytte, U.P.; Wormald, M.R.; Dwek, R.A.; Fleet, G.W.J. 1,2:3,4-Di-O-isopropylidene-α-d-tagatofuranose. Acta Crystallogr. Sect. E 2005, 61, o2891–o2893. [Google Scholar] [CrossRef]
- Yoshihara, A.; Haraguchi, S.; Gullapalli, P.; Rao, D.; Morimoto, K.; Takata, G.; Jones, N.; Jenkinson, S.F.; Wormald, M.R.; Dwek, R.A.; et al. Isomerization of deoxyhexoses: Green bioproduction of 1-deoxy-d-tagatose from l-fucose and of 6-deoxy-d-tagatose from d-fucose using Enterobacter agglomerans strain 221e. Tetrahedron: Asymmetry 2008, 19, 739–745. [Google Scholar] [CrossRef]
- Baráth, M.; Lin, C.-H.; Tvaroška, I.; Hirsch, J. Development of transition state analogue inhibitors for N-acetylglycosyltransferases bearing d-psico- or d-tagatofuranose scaffolds. Chem. Pap. 2015, 69, 348–357. [Google Scholar] [CrossRef]
- Makura, Y.; Ueda, A.; Matsuzaki, T.; Minamino, T.; Tanaka, M. α-Selective glycosidation of d-tagatofuranose with a 3,4-O-isopropylidene protection. Tetrahedron 2019, 75, 3758–3766. [Google Scholar] [CrossRef]
- Hu, Y.; Iyoshi, A.; Makura, Y.; Tanaka, M.; Ueda, A. 1,3,4,5-Tetra-O-benzoyl-α-d-tagatopyranose. Molbank 2025, 2025, M2041. [Google Scholar] [CrossRef]
- Angyal, S.J.; Bethell, G.S. Conformational analysis in carbohydrate chemistry. III* The 13C N.M.R. spectra of the hexuloses. Aust. J. Chem. 1976, 29, 1249–1265. [Google Scholar] [CrossRef]
- Takagi, S.; Rosenstein, R.D. The structure of α-d-tagatose and comparison with crystal structures of other ketohexoses. Carbohydr. Res. 1969, 11, 156–158. [Google Scholar] [CrossRef]
- Punzo, F.; Watkin, D.J.; Fleet, G.W.J. α-d-Tagatopyranose. Acta Crystallogr. Sect. E 2009, 65, o1393–o1394. [Google Scholar] [CrossRef] [PubMed]
- Miura, D.; Fujimoto, T.; Tashiro, M.; Machinami, T. Crystal Structure of 1,3,4,5-Tetra-O-acetyl-α-d-tagatopyranose. X-Ray Struct. Anal. Online 2014, 30, 3–4. [Google Scholar] [CrossRef]
- Khouvine, Y.; Arragon, G.; Tomoda, Y. Ketoses. II. The structure of α-d-tagatose. Bull. Soc. Chim. Fr. Mem. 1939, 6, 354–359. [Google Scholar]
- Angyal, S.; Bodkin, C.; Mills, J.; Pojer, P. Complexes of carbohydrates with metal cations. IX. Synthesis of the methyl d-tagatosides, d-psicosides, d-apiosides and d-erythrosides. Aust. J. Chem. 1977, 30, 1259–1268. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, R.U.; Koto, S.; Voisin, D. The Exo-Anomeric Effect. ACS Symp. Ser. 1979, 87, 17–29. [Google Scholar] [CrossRef]
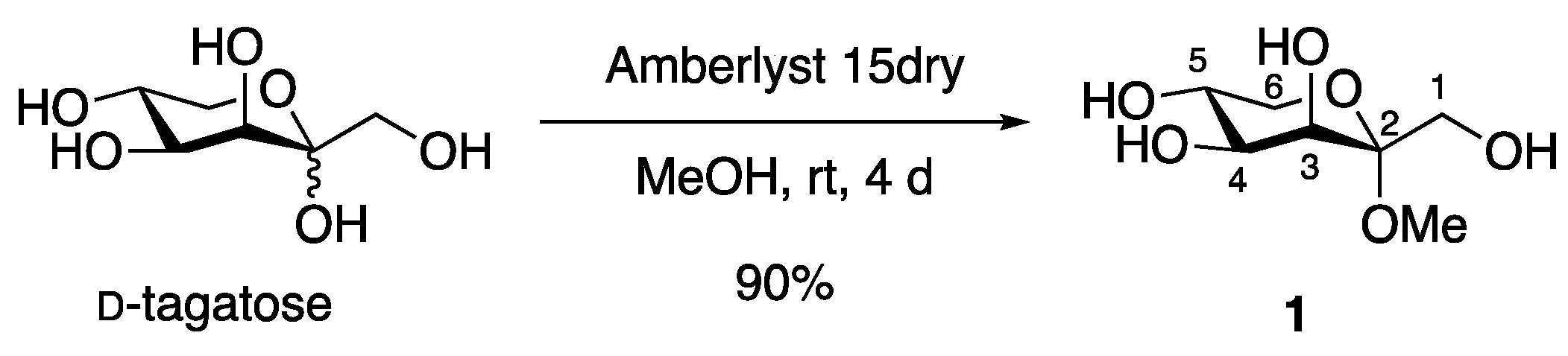
| Donor | Acceptor | Distance (Å) | Angle (°) | Symmetry | ||
|---|---|---|---|---|---|---|
| D–H | A | D–H | H···A | D···A | D–H···A | operations |
| O1-H | O2′ | 0.840 | 1.927 | 2.760 | 170.9 | –1/2+x, 1/2−y, 1−z |
| O3-H | O5′ | 0.840 | 1.982 | 2.814 | 171.0 | x, −1+y, z |
| O4-H | O6′ | 0.840 | 1.902 | 2.732 | 169.5 | 1+x, y, z |
| O5-H | O4′ | 0.852 | 1.871 | 2.680 | 157.8 | 2−x, 1/2+y, 1/2−z |
| Description | Torsion Angles (°) | |
|---|---|---|
| 1 | 2 1 | |
| O1-C1-C2-O2 | –49.5 | 50.8 |
| O2-C2-C3-O3 | 177.6 | 174.6 |
| O3-C3-C4-O4 | –57.2 | –57.9 |
| O4-C4-C5-O5 | –71.6 | –62.1 |
| O5-C5-C6-O6 | –169.3 | –175.7 |
| ϕ (O6-C2-O2-CH3) | 61.1 | N/A 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Iyoshi, A.; Tanaka, M.; Ueda, A. Methyl α-d-Tagatopyranoside. Molbank 2025, 2025, M2046. https://doi.org/10.3390/M2046
Hu Y, Iyoshi A, Tanaka M, Ueda A. Methyl α-d-Tagatopyranoside. Molbank. 2025; 2025(3):M2046. https://doi.org/10.3390/M2046
Chicago/Turabian StyleHu, Yiming, Akihiro Iyoshi, Masakazu Tanaka, and Atsushi Ueda. 2025. "Methyl α-d-Tagatopyranoside" Molbank 2025, no. 3: M2046. https://doi.org/10.3390/M2046
APA StyleHu, Y., Iyoshi, A., Tanaka, M., & Ueda, A. (2025). Methyl α-d-Tagatopyranoside. Molbank, 2025(3), M2046. https://doi.org/10.3390/M2046





