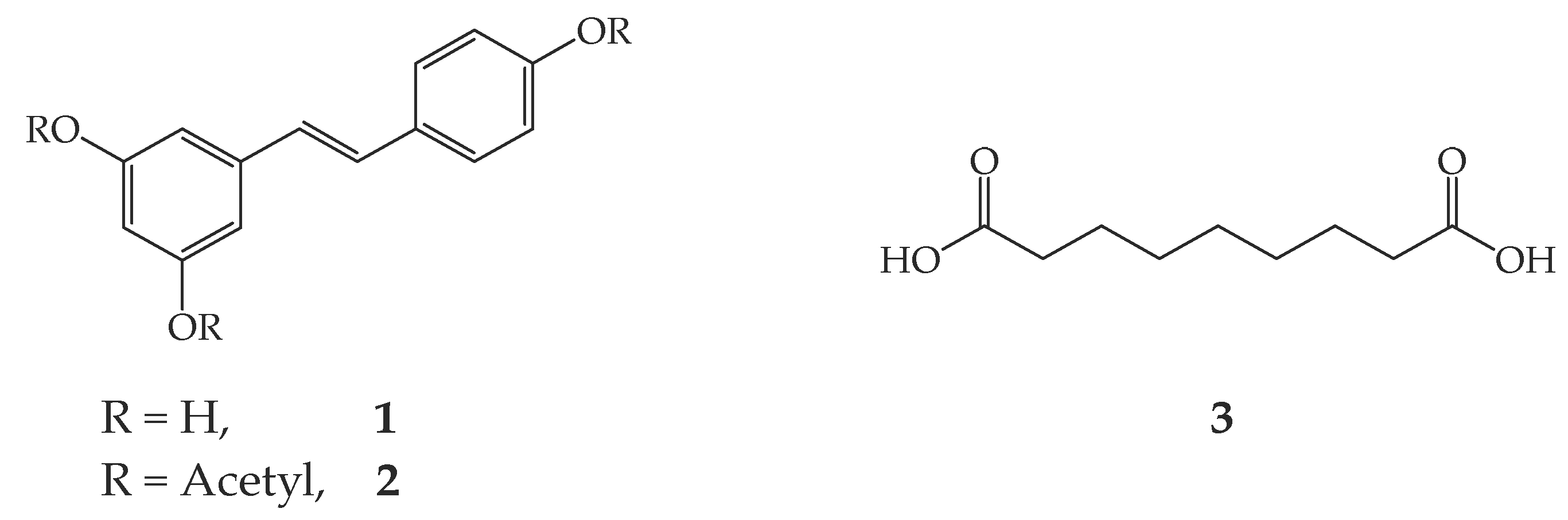
Bis(4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate
Abstract
1. Introduction
2. Results
3. Materials and Methods
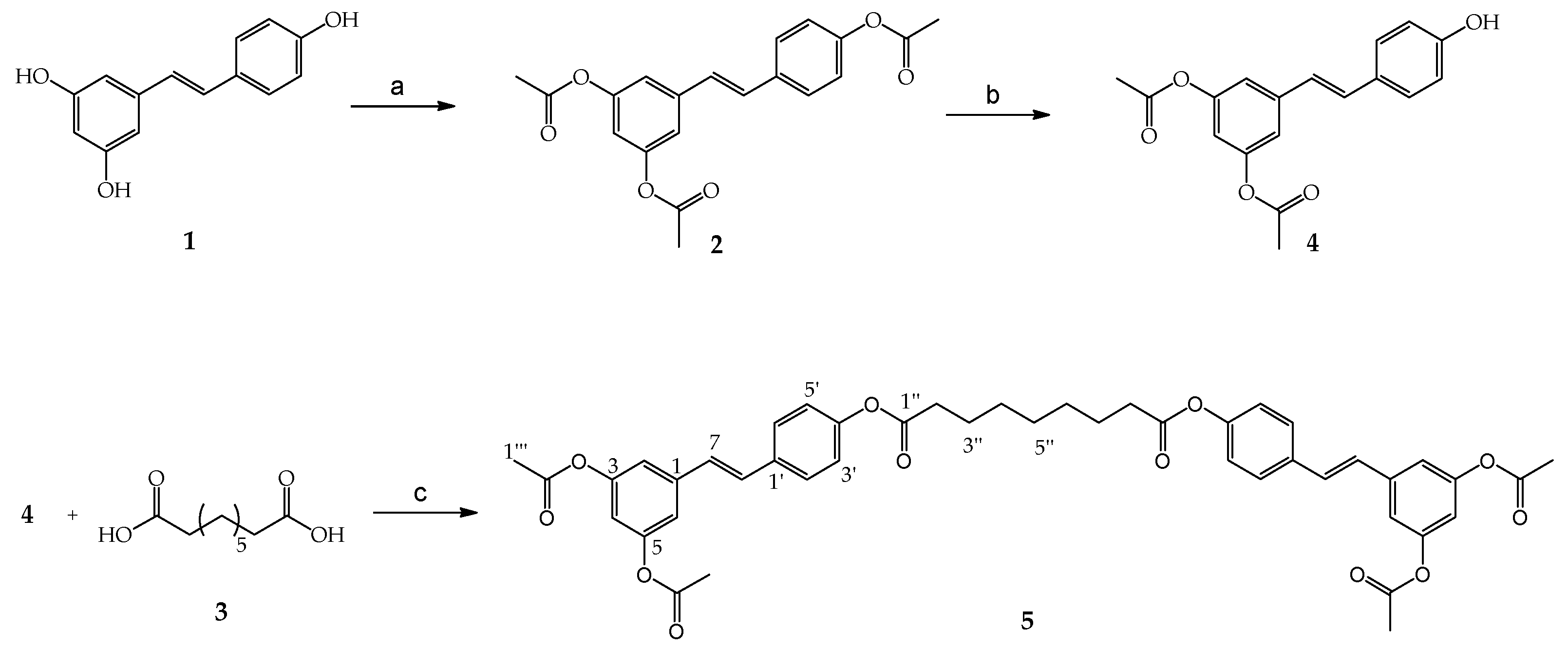
3.1. Synthesis of (E)-5-(4-Acetoxy Styryl)-1,3-Phenylene Diacetate (2)
3.2. Synthesis of (E)-5-(4-Hydroxystyryk)-1,3-Phenylene Diacetate (4)
3.3. Synthesis of Bis (4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bejenaru, L.; Bita, A.; Belu, I.; Segneanu, A.; Radu, A.; Dumitru, A.; Ciocilteu, M.; Mogosanu, G.; Bejenaru, C. Resveratrol: A Review on the Biological Activity and Applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Tiwari, R.; Tiwari, G.; Ramachandran, V. Resveratrol: A Vital Therapeutic Agent with Multiple Health Benefits. Drug Res. 2022, 72, 5–17. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedcines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics 2025, 17, 134. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Durazzo, A.; Lucarini, M.; Souto, E.; Santini, A.; Imran, M.; Moussa, A.; Mostafa, N.; El-Shazly, M.; et al. Resveratrol’ biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J. Herb. Med. 2022, 32, 100550. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Al-Johani, N.S.; Alhosani, N.M.; Alkeraishan, N.; Alhenaky, A.; Abd-Elkander, O.H.; El-Seedi, H.R.; et al. Resveratrol and Neuroprotection: Impacy and its therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 30, 619024. [Google Scholar]
- Ahmadi, R.; Ebrahimzadeh, M. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.; Tringali, C. Resveratrol-Related Polymethoxystilbene Glycosides: Synthesis, Antiproliferative Activity, and Glycosidase Inhibition. J. Nat. Prod. 2015, 78, 2675–2683. [Google Scholar] [CrossRef]
- Chillemi, R.; Cardullo, N.; Greco, V.; Malfa, G.; Tomasello, B.; Sciuto, S. Syntesis of amphiphilic resveratrol lipoconjugates and evaluation of their anticancer activity towards neuroblastoma SH-SY5Y cell line. Eur. J. Med. Chem. 2015, 96, 467–481. [Google Scholar] [CrossRef]
- Pyo, I.; Yun, S.; Yoon, Y.; Choi, J.; Lee, S. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Sciacca, C.; Cardullo, N.; Pulvirenti, L.; Travagliante, G.; D’Urso, A.; D’Agata, R.; Peri, E.; Cancemi, P.; Cornu, A.; Deffieux, D.; et al. Syntesis of obovatol and related neolignan analoguess as α-glucosidase and α-amylase inhibitors. Bioorg. Chem. 2024, 147, 107392. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, C.; Cardullo, N.; Pulvirenti, L.; Di Francesco, A.; Muccilli, V. Evaluation of honokiol, magnolol and of a library of new nitrogenated neolignans as pancreatic lipase inhibitors. Bioorg. Chem. 2023, 134, 106455. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Marengo, M.; Parravicini, C.; Eberini, I.; Dallavalle, S.; Bonomi, F.; Iametti, S.; Pinto, A. Inhibiton of pancreatic α-amylase by Resveratrol Derivatives:Biological Activity and Molecular Modelling Evidence for Cooperativity berween Viniferin Enantiomers. Molecules 2019, 24, 3225. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Phan, M.H.; Thai, T.T.; Lam, T.P.; Lai, N.V.T.; Nguyen, T.T.; Nguyen, T.V.P.; Vo, C.V.T.; Thai, K.M.; Tran, T.-D. Discovery of novel flavonoid derivatives as potential dual inhibitors against α-glucosidase and α-amylase: Virtual screening, synthesis and biological evaluation. Mol. Divers. 2024, 28, 1629–1650. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Z.; Takano, H.; Xu, Z.; Nishiwaki, H.; Yonekura, L.; Yang, R.; Tamura, H. Rosmarinic acid and its ester derivaties for enhancing antibactertial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J. Food Biochem. 2019, 43, e12719. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Kim, J.Y.; Arooj, M.; Kim, S.; Hwang, S.; Kim, B.W.; Park, K.H.; Lee, K.W. Binding Mode Analyses and Pharmacophore Model Development for Stilbene Derivates as a Novel and Competitive Class of α-Glucosidase Inhibitors. PLoS ONE 2014, 9, e85827. [Google Scholar]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α- amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Franco, D.; de Carvalho, G.; Rocha, P.; Teixeira, R.; da Silva, A.; Raposo, N. Inhibitory Effects of Resveratrol Analogs on Mushroom Tyrosinase Activity. Molecules 2012, 17, 11816–11825. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Liu, L.; Wang, F.; Ouyang, L.; Zhang, L.; Hu, X.; Wang, G. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef]
- Na, J.; Shin, J.; Choi, H.; Kwon, S.; Park, K. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.; Younes, S.; Abou Chahla, M.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.; Suh, H.; Lee, I.; Koh, J.; Boo, Y. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.; Oslizlo, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karlowicz-Bodalska, K. The multiple uses of azelaic acid in dermatology: Mechanism of action, preparations, and potential therapeutic applications. Postepy Dermatol. Alergol. 2023, 40, 716–724. [Google Scholar] [CrossRef]
- Schallreuter, K.; Wood, J. A possible mechanism of action for azelaic acid in the human epidermis. Arch. Dermatol. Res. 1990, 282, 168–171. [Google Scholar] [CrossRef]
- Schulte, B.; Wu, W.; Rosen, T. Azelaic Acid: Evidence-based Update on Mechanism of Action and Clinical Application. JDD 2015, 14, 964–968. [Google Scholar]
- Nicolosi, G.; Spatafora, C.; Tringali, C. Chemo-enzymatic preparation of resveratrol derivatives. J. Mol. Catal. B Enzym. 2002, 16, 223–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacca, C.; Grasso, G.M.; Cardullo, N.; Muccilli, V. Bis(4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate. Molbank 2025, 2025, M2044. https://doi.org/10.3390/M2044
Sciacca C, Grasso GM, Cardullo N, Muccilli V. Bis(4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate. Molbank. 2025; 2025(3):M2044. https://doi.org/10.3390/M2044
Chicago/Turabian StyleSciacca, Claudia, Giulia Maria Grasso, Nunzio Cardullo, and Vera Muccilli. 2025. "Bis(4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate" Molbank 2025, no. 3: M2044. https://doi.org/10.3390/M2044
APA StyleSciacca, C., Grasso, G. M., Cardullo, N., & Muccilli, V. (2025). Bis(4-((E)-3,5–Diacetoxystyryl)phenyl)nonanedioate. Molbank, 2025(3), M2044. https://doi.org/10.3390/M2044





