4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl and Its Corresponding Stable Diradical
Abstract
1. Introduction
2. Results and Discussion
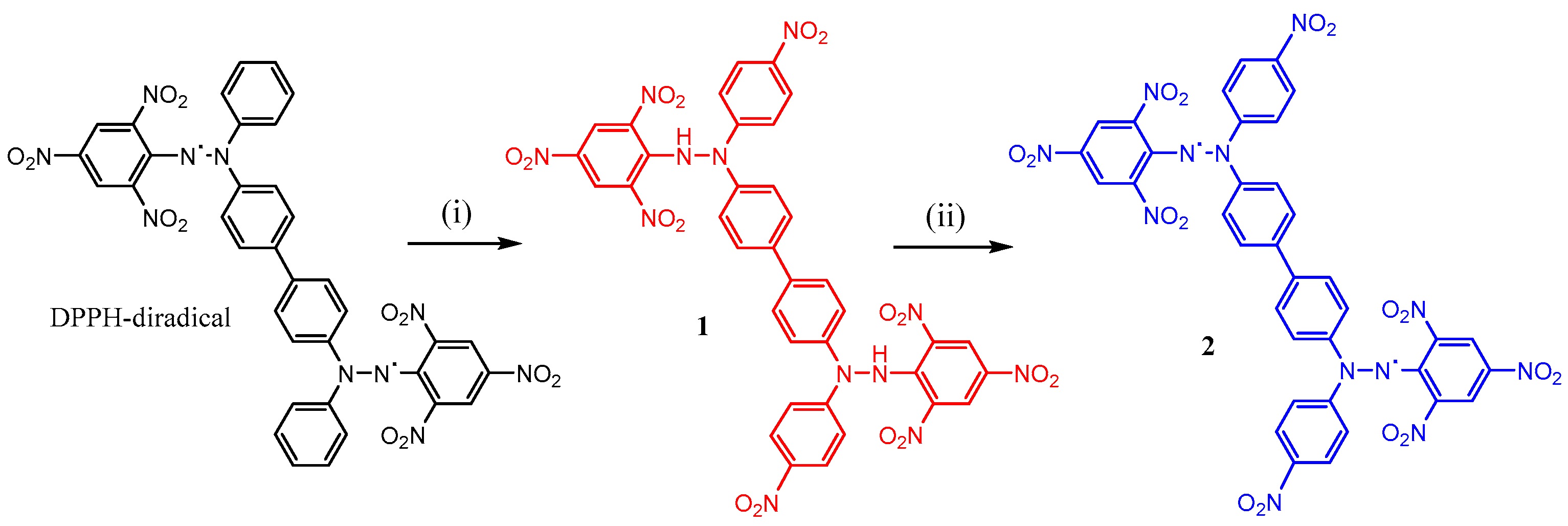
2.1. Synthesis
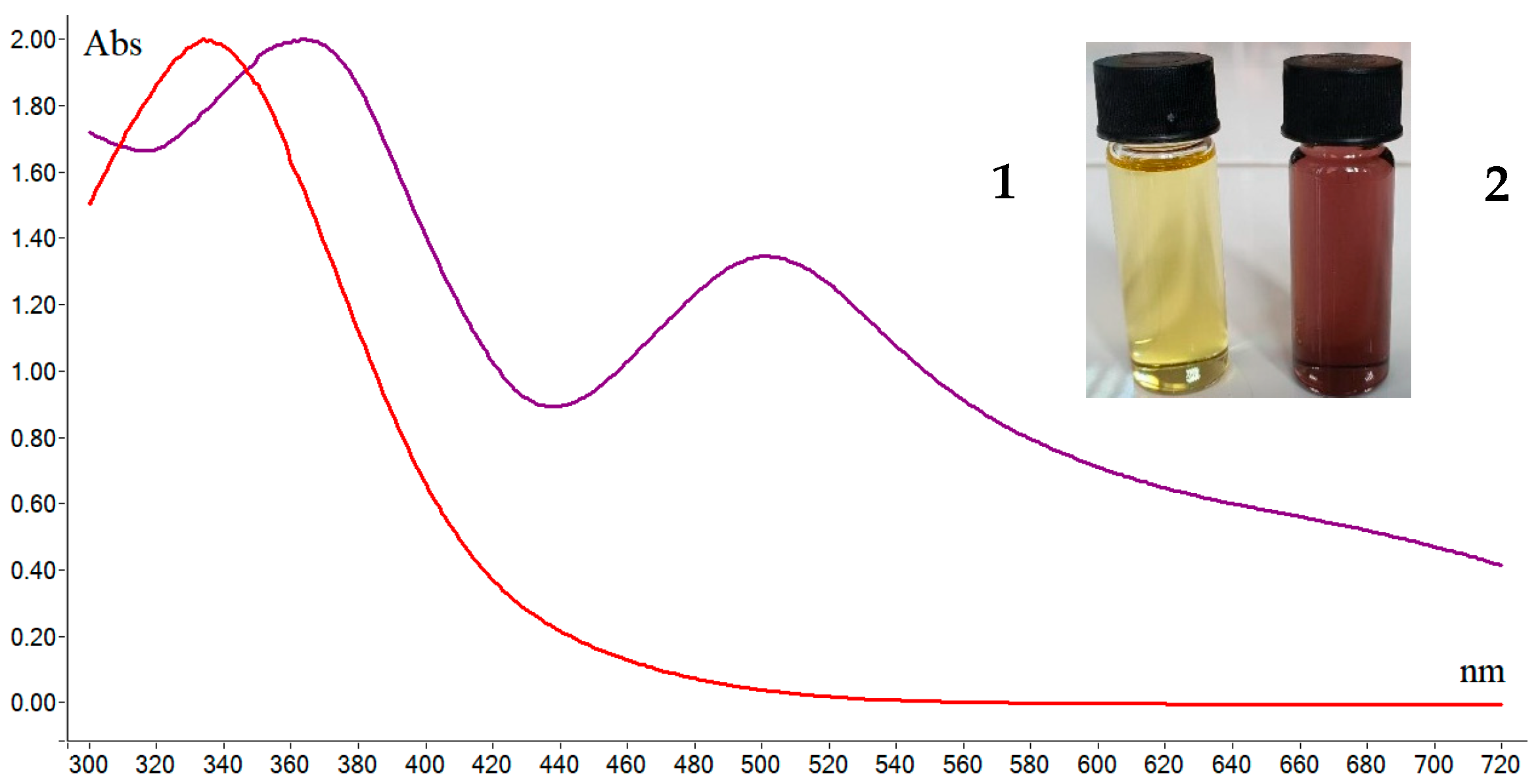
2.2. Characterization of Compounds 1 and 2
3. Materials and Methods
3.1. Compound 1, 4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl
3.2. Compound 2, 4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazyl)-1,1′-biphenyl Diradical
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chechik, V.; Carter, E.; Murphy, D.M. Electron Paramagnetic Resonance; Oxford Chemistry Primers; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Ionita, P. The Chemistry of DPPH Free Radical and Congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Fidalgo-Marijuan, A.; Dias, J.C.; Gonçalves, R.; Salado, M.; Costa, C.M.; Lanceros-Méndez, S. Molecular design of functional polymers for organic radical batteries. Energy Storage Mater. 2023, 60, 102841. [Google Scholar] [CrossRef]
- Friebe, C.; Zens, C.; Kupfer, S.; Schubert, U.S. Additive-Free Organic Radical Batteries Prepared through Electrochemical Polymerization of TEMPO-Decorated Terthiophene. J. Phys. Chem. C 2023, 127, 1333–1344. [Google Scholar] [CrossRef]
- Howey, D.A.; Roberts, S.A.; Viswanathan, V.; Mistry, A.; Beuse, M.; Khoo, E.; DeCaluwe, S.C.; Sulzer, V. Free Radicals: Making a Case for Battery Modeling. Electrochem. Soc. Interface 2020, 29, 30. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Easley, A.D.; Kang, N.; Khan, S.; Lim, S.-M.; Rezenom, Y.H.; Wang, S.; Tran, D.K.; Fan, J.; Letteri, R.A.; et al. Polypeptide organic radical batteries. Nature 2021, 593, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, S.; Renn, K. Zweiwertiger Stickstoff: Über das α,α-Diphenyl-β-trinitrophenylhydrazyl. Ber. Dtsch. Chem. Ges. 1922, 55, 628–643. [Google Scholar] [CrossRef]
- Chen, O.; Zhuang, J.; Guzzetta, F.; Lynch, J.; Angerhofer, A.; Cao, Y.C. Synthesis of water-soluble 2,2′-diphenyl-1-picrylhydrazyl nanoparticles: A new standard for electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 2009, 131, 12542–12543. [Google Scholar] [CrossRef] [PubMed]
- Dobre, A.F.; Lete, C.; Kuncser, V.E.; Iacob, N.; Madalan, A.M.; Ionita, G.; Harada, M.; Kitagawa, Y.; Ionita, P. The Dimer of the DPPH Stable Radical. ACS Omega 2025, 10, 36662–36671. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A. m-Quinodimethane-Based Fused-Ring Diradicals with Singlet and Triplet Ground States. Chemistry 2025, 7, 40. [Google Scholar] [CrossRef]
- Yamada, S.; Shigemoto, I.; Kawakami, T.; Isobe, H.; Shoji, M.; Miyagawa, K.; Yamaguchi, K. Quantum Mechanical Approaches to Strongly Correlated Electron Systems: Structure, Bonding, and Properties of Diradicals, Triradicals, and Polyradicals. Chemistry 2025, 7, 38. [Google Scholar] [CrossRef]
- Betkhoshvili, S.; Poater, J.; Moreira, I.d.P.R.; Bofill, J.M. Fully Conjugated Heteroatomic Non- and Quasi-Alternant Polyradicals. Chemistry 2025, 7, 45. [Google Scholar] [CrossRef]
- Ionita, P.; Caproiu, M.T.; Luca, C.; Constantinescu, T.; Caldararu, H.; Balaban, A.T. Selective (15N) nitration of 2,2-diphenyl-1-(2,4- or 2,6-dinitrophenyl)-hydrazines or -hydrazyls. J. Label. Comp. Radiopharm. 1998, 41, 791–799. [Google Scholar] [CrossRef]
- Weil, J.A.; Sane, K.V.; Kinkade, J.M. The reaction between 2,2-diphenyl-1-picrylhydrazyl and nitrogen dioxide. J. Phys. Chem. 1961, 65, 710–712. [Google Scholar] [CrossRef]
- Available online: https://www.niehs.nih.gov/research/resources/software/tox-pharm/tools (accessed on 1 June 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caproiu, M.T.; Ionita, P. 4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl and Its Corresponding Stable Diradical. Molbank 2025, 2025, M2045. https://doi.org/10.3390/M2045
Caproiu MT, Ionita P. 4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl and Its Corresponding Stable Diradical. Molbank. 2025; 2025(3):M2045. https://doi.org/10.3390/M2045
Chicago/Turabian StyleCaproiu, Miron T., and Petre Ionita. 2025. "4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl and Its Corresponding Stable Diradical" Molbank 2025, no. 3: M2045. https://doi.org/10.3390/M2045
APA StyleCaproiu, M. T., & Ionita, P. (2025). 4,4′-Bis(1-(4-nitrophenyl)-2-(2,4,6-trinitrophenyl)hydrazineyl)-1,1′-biphenyl and Its Corresponding Stable Diradical. Molbank, 2025(3), M2045. https://doi.org/10.3390/M2045






