6-Amino-4-phenylpyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitrile
Abstract
1. Introduction
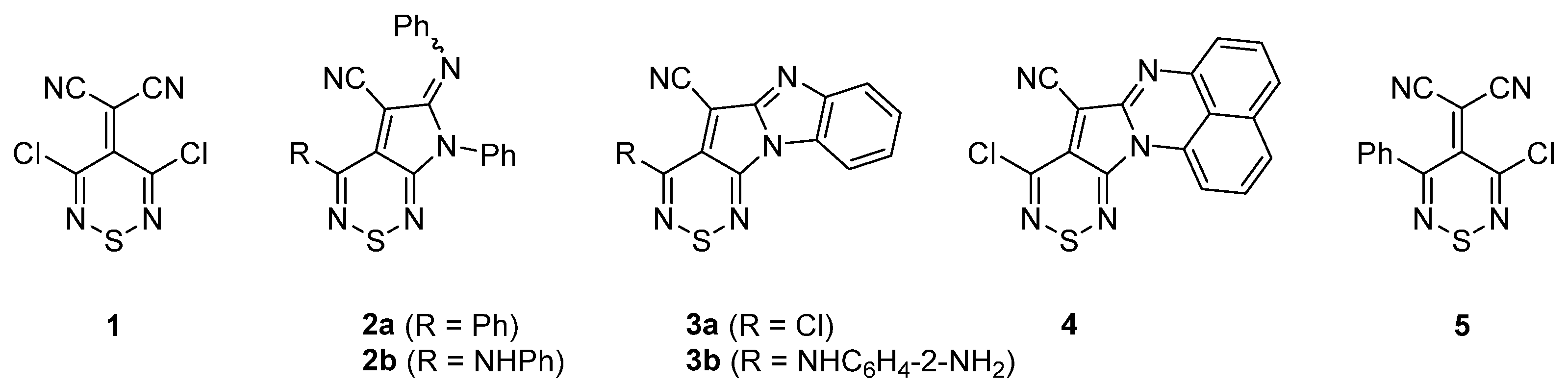
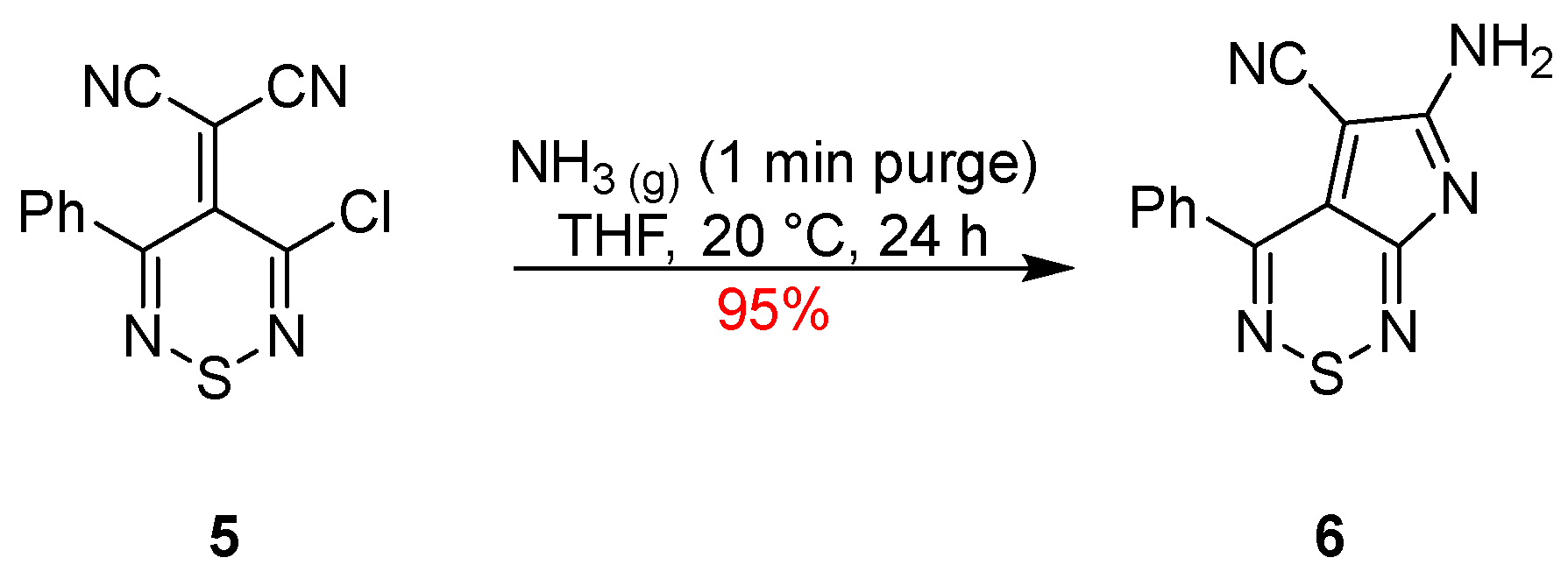
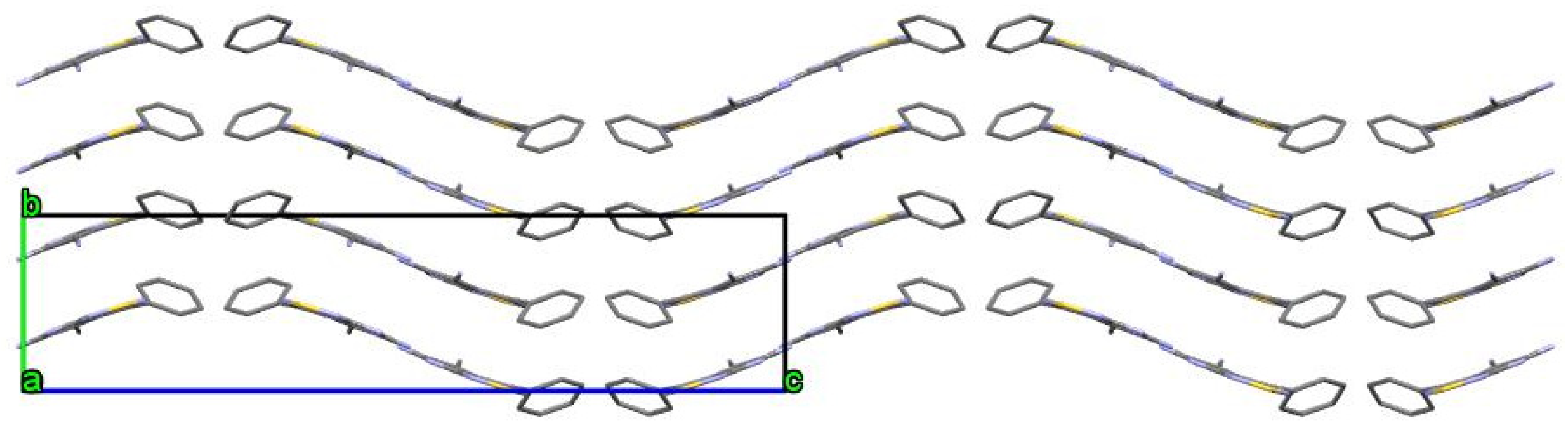
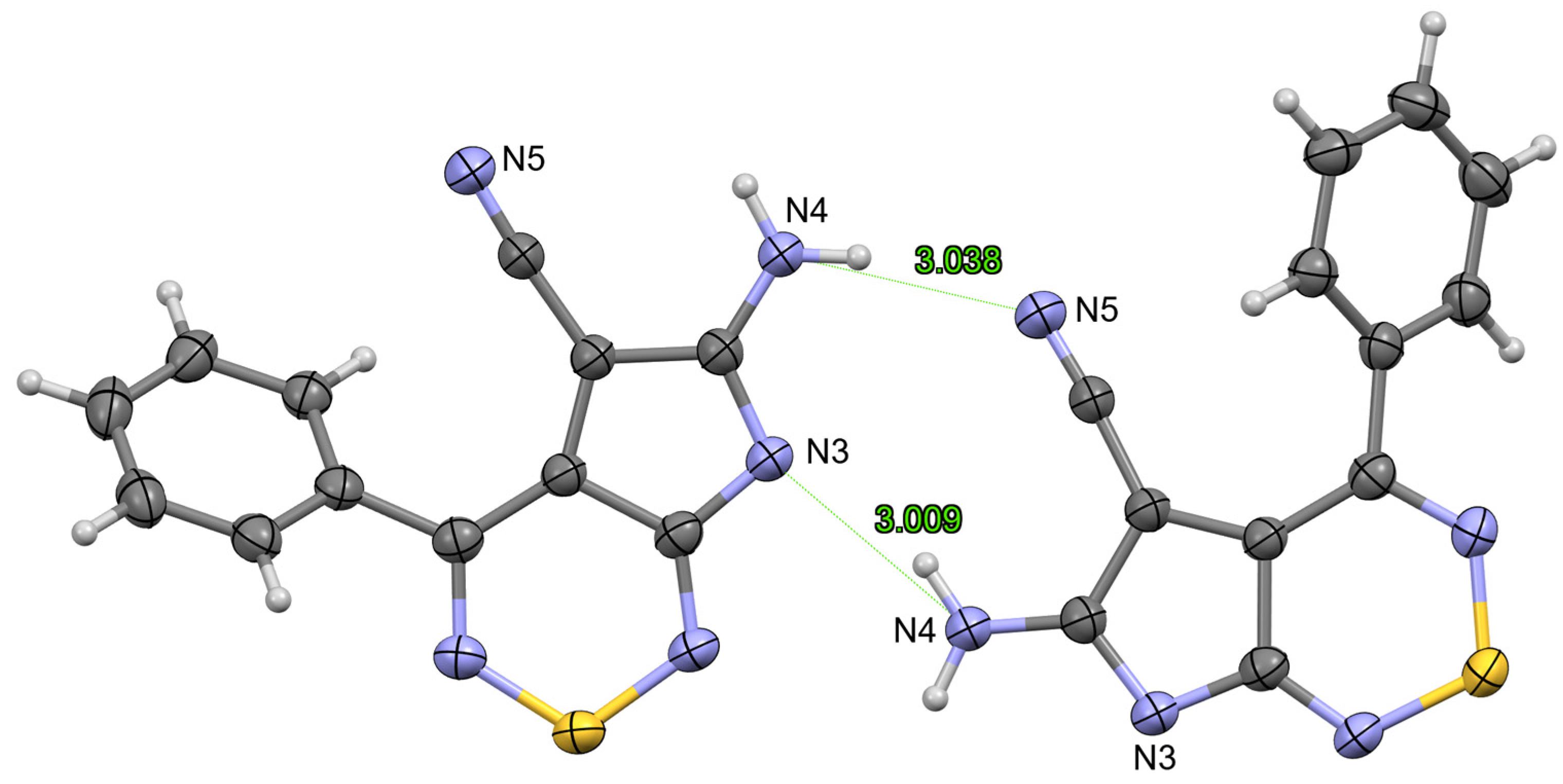
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarganes-Tzitzikas, T.; Neochoritis, C.G.; Dömling, A. Atorvastatin (Lipitor) by MCR. ACS Med. Chem. Lett. 2019, 10, 389–392. [Google Scholar] [CrossRef]
- Wong, S.; Gardocki, J.F.; Pruss, T.P. Pharmacologic evaluation of Tolectin (tolmetin, McN-2559) and McN-2891, two anti-inflammatory agents. J. Pharmacol. Exp. Ther. 1973, 185, 127–138. [Google Scholar] [CrossRef]
- Sawyers, C.L. Finding the next Gleevec: FLT3 targeted kinase inhibitor therapy for acute myeloid leukemia. Cancer Cell 2002, 1, 413–415. [Google Scholar] [CrossRef]
- de Lacy Costello, B.P.J.; Evans, P.; Guernion, N.; Ratcliffe, N.M.; Sivanand, P.S.; Teare, G.C. The synthesis of a number of 3-alkyl and 3-carboxy substituted pyrroles; their chemical polymerisation onto poly(vinylidene fluoride) membranes, and their use as gas sensitive resistors. Synth. Met. 2000, 114, 181–188. [Google Scholar] [CrossRef]
- Xue, T.; Tang, L.; Tang, R.; Li, Y.; Nie, J.; Zhu, X. Color evolution of a pyrrole-based enone dye in radical photopolymerization formulations. Dyes Pigm. 2021, 188, 109212. [Google Scholar] [CrossRef]
- Sha, Q.; Arman, H.; Doyle, M.P. Three-Component Cascade Reactions with 2,3-Diketoesters: A Novel Metal-Free Synthesis of 5-Vinyl-pyrrole and 4-Hydroxy-indole Derivatives. Org. Lett. 2015, 17, 3876–3879. [Google Scholar] [CrossRef]
- Dutta, I.; Rachuri, Y.; Gonçalves, T.P.; Huang, M.H.; Huang, K.-W. Pyrroles and Their Benzo Derivatives: Structure. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 3, Chapter 3.01; pp. 1–67. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A. Pyrroles and Their Benzo Derivatives: Applications. In Comprehensive Heterocyclic Chemistry IV; StC Black, D., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 3, Chapter 3.04; pp. 175–189. [Google Scholar] [CrossRef]
- Van Order, R.B.; Lindwall, H.G. Indole. Chem. Rev. 1942, 30, 69–96. [Google Scholar] [CrossRef]
- Yakhontov, L.N. Condensed heteroaromatic systems that include π-surplus and π-deficient rings. Azaindoles (review). Chem. Heterocycl. Compd. 1982, 18, 873–885. [Google Scholar] [CrossRef]
- Amarnath, V.; Madhav, R. A survey of methods for the preparation of pyrrolopyrimidines. Synthesis 1974, 1974, 837–859. [Google Scholar] [CrossRef]
- Blaise, E.; Kümmerle, A.E.; Hammoud, H.; de Araújo-Júnior, J.X.; Bihel, F.; Bourguignon, J.-J.; Schmitt, M. Access to 4-Alkylaminopyridazine Derivatives via Nitrogen-Assisted Regioselective Pd-Catalyzed Reactions. J. Org. Chem. 2014, 79, 10311–10322. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Chemistry of 4-dicyanomethylene-1,2,6-thiadiazines. J. Chem. Soc., Perkin Trans. 1 2000, 1081–1088. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Cyclisation chemistry of 4H-1,2,6-thiadiazines. J. Chem. Soc., Perkin Trans. 1 2000, 2601–2607. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. Synthesis of 2-(4H-1,2,6-thiadiazin-4-ylidene)malononitriles. Tetrahedron 2014, 70, 8334–8342. [Google Scholar] [CrossRef]
- Waver, I. Hindered rotation in amidines. J. Mol. Struct. 1990, 218, 165–167. [Google Scholar] [CrossRef]
- Emelina, E.E.; Petrov, A.A.; Filyukov, D.I. Tautomerism of 3(5)-amino-4-cyano and 3(5)-amino-4-thiocyanatopyrazoles. Russ. J. Org. Chem. 2001, 37, 1348–1350. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. One Pot Synthesis of 3-Amino-1H-pyrazole-4-carbonitrile. Monatsh. Chem. 1981, 112, 875–878. [Google Scholar] [CrossRef]
- Andrianov, V.G.; Eremeev, A.V. Synthesis and properties of 4-amino-3-cyanofurazan. Chem. Heterocycl. Compd. 1994, 30, 608–611. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Zhang, M.-X.; Zuckerman, N.B.; DeHope, A.J.; Parrish, D.A. Synthesis and characterization of multicyclic oxadiazoles and 1-hydroxytetrazoles as energetic materials. Chem. Heterocycl. Compd. 2017, 53, 760–778. [Google Scholar] [CrossRef]
- Martin, N.H.; Nance, K.H. Modeling through-space magnetic shielding over ethynyl, cyano, and nitro groups. J. Mol. Graph. Model. 2002, 21, 51–56. [Google Scholar] [CrossRef]
- Ehnbom, A.; Hall, M.B.; Gladysz, J.A. Origin of Shielding and Deshielding Effects in NMR Spectra of Organic Conjugated Polyynes. Org. Lett. 2019, 21, 753–757. [Google Scholar] [CrossRef]
- Bruker. Apex3, Saint; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Cryst. 2015, 71, 59–75. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. 6-Amino-4-phenylpyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitrile. Molbank 2025, 2025, M2043. https://doi.org/10.3390/M2043
Kalogirou AS, Kourtellaris A, Koutentis PA. 6-Amino-4-phenylpyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitrile. Molbank. 2025; 2025(3):M2043. https://doi.org/10.3390/M2043
Chicago/Turabian StyleKalogirou, Andreas S., Andreas Kourtellaris, and Panayiotis A. Koutentis. 2025. "6-Amino-4-phenylpyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitrile" Molbank 2025, no. 3: M2043. https://doi.org/10.3390/M2043
APA StyleKalogirou, A. S., Kourtellaris, A., & Koutentis, P. A. (2025). 6-Amino-4-phenylpyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitrile. Molbank, 2025(3), M2043. https://doi.org/10.3390/M2043





