Synthesis and Structural Characterization of Manganese(I) Complexes Ligated by 2-Azabutadienes (ArS)2C=C(H)-N=CPh2
Abstract
1. Introduction
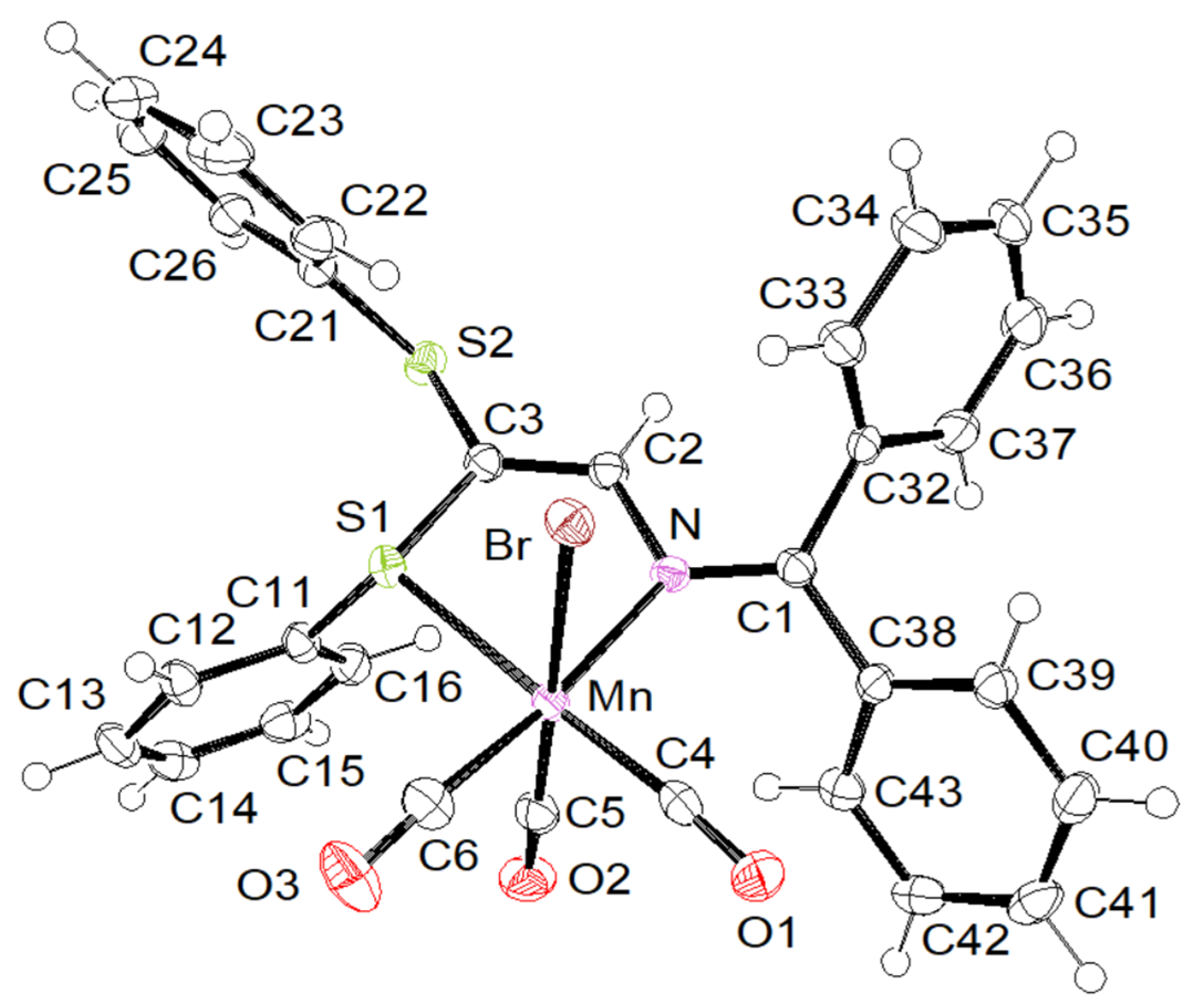
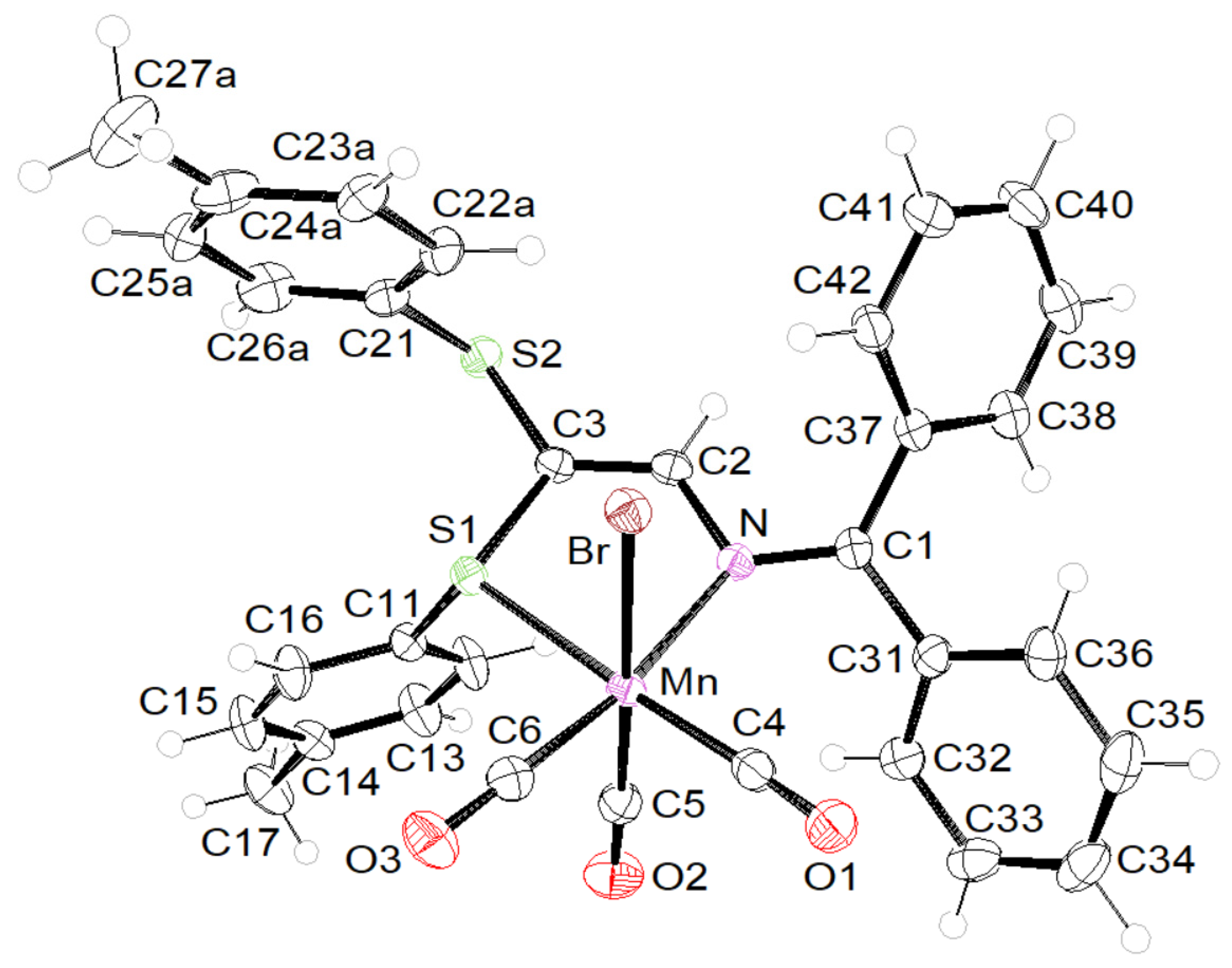
2. Results and Discussion
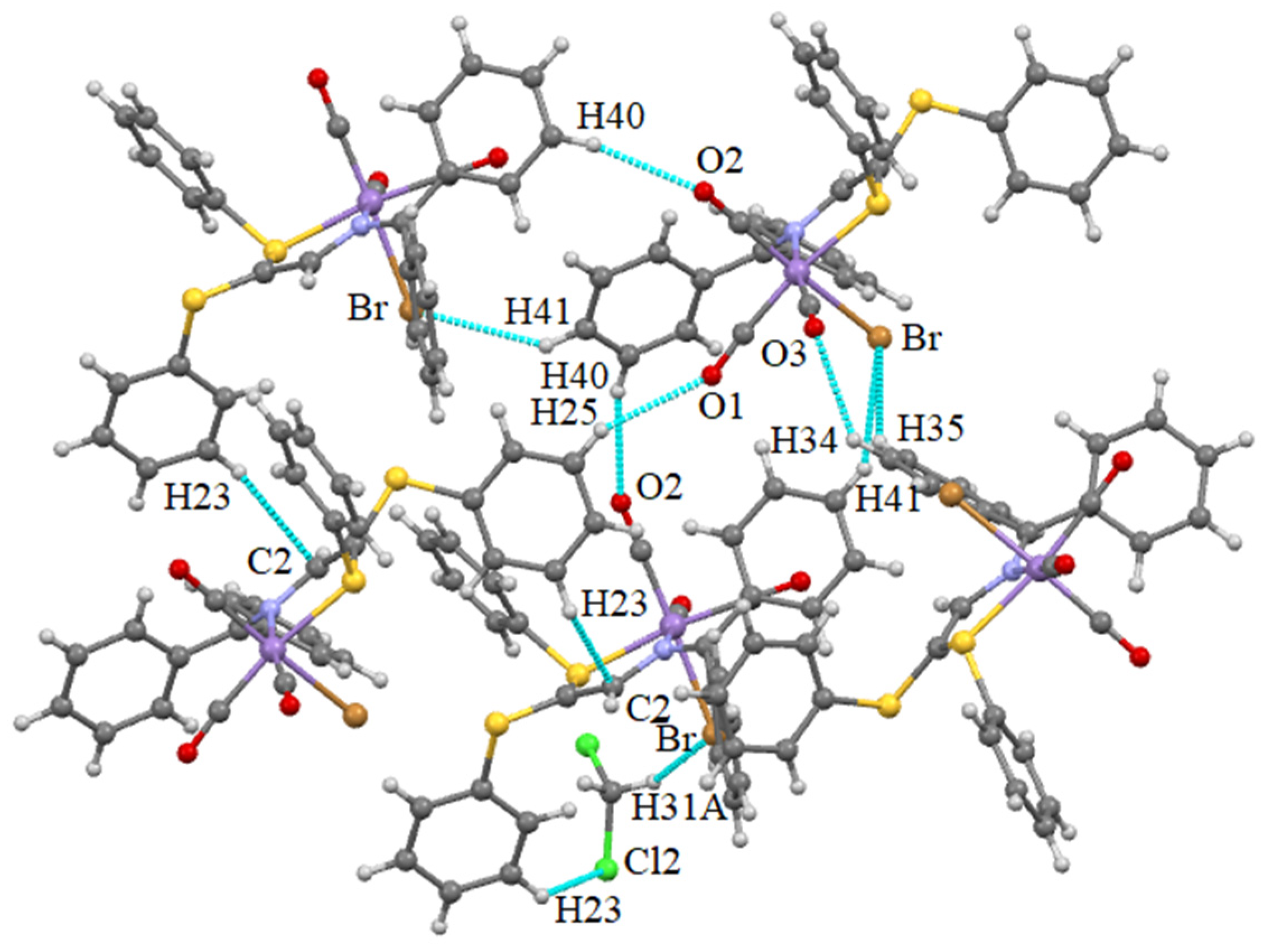
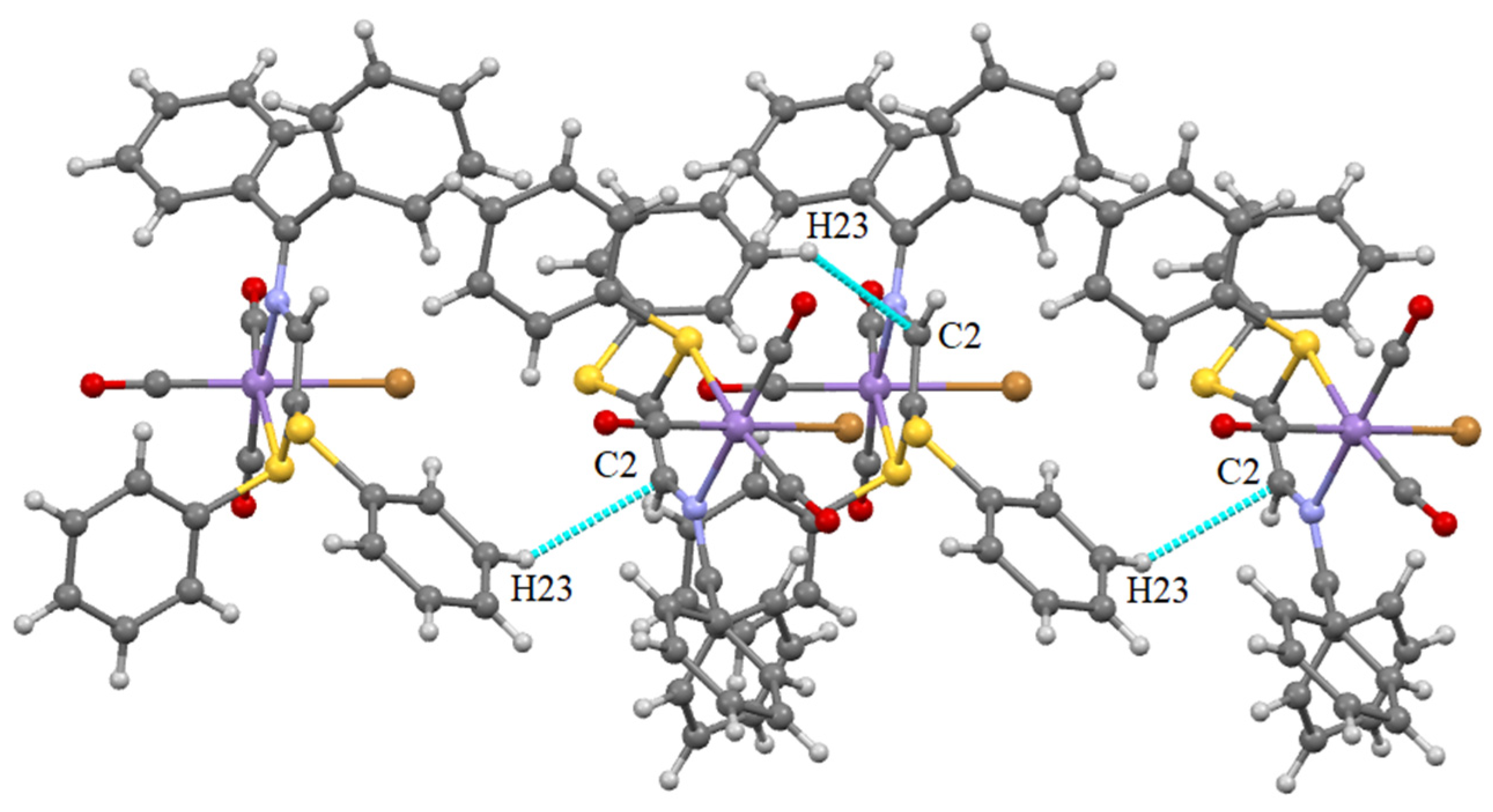
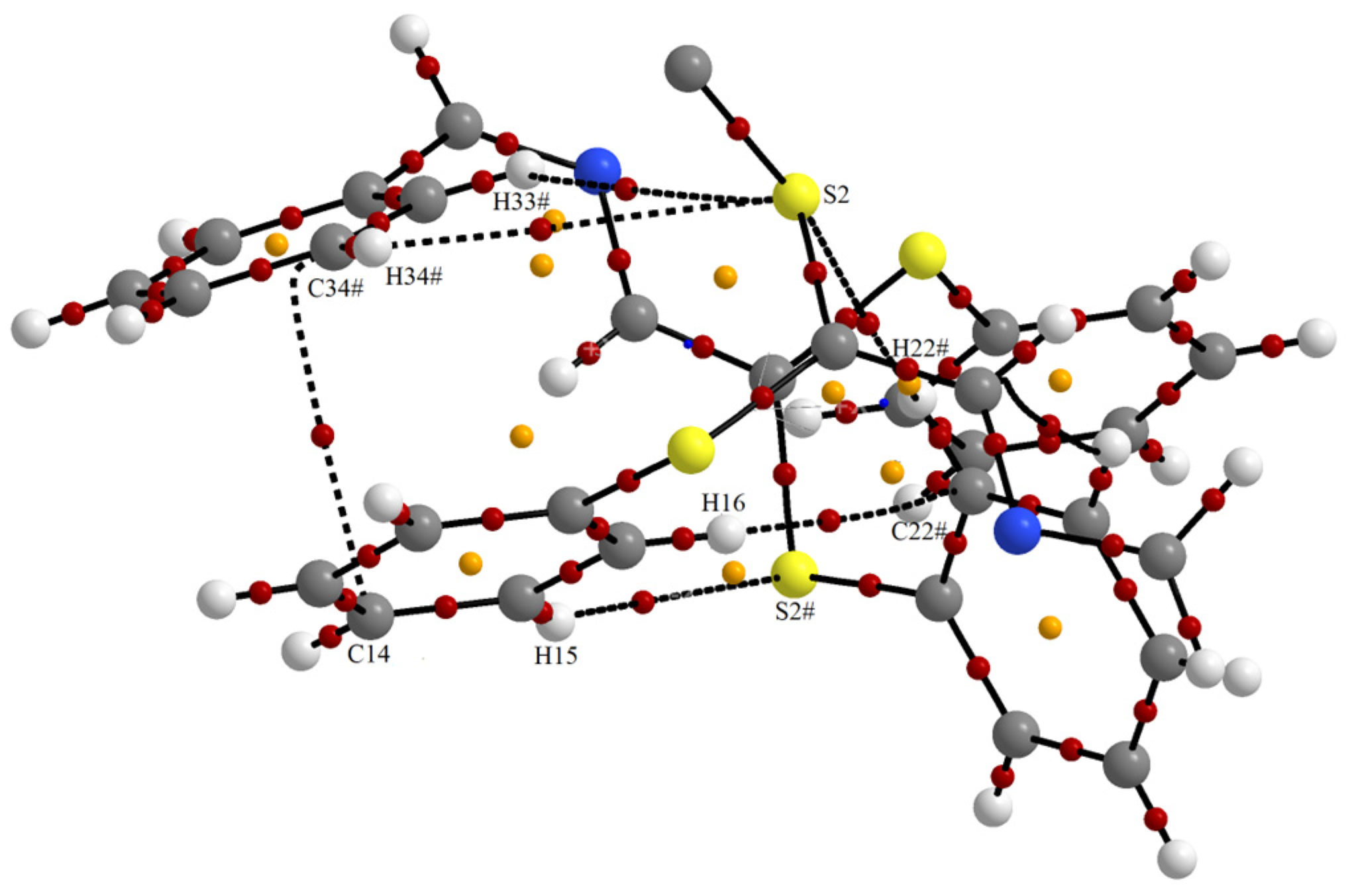
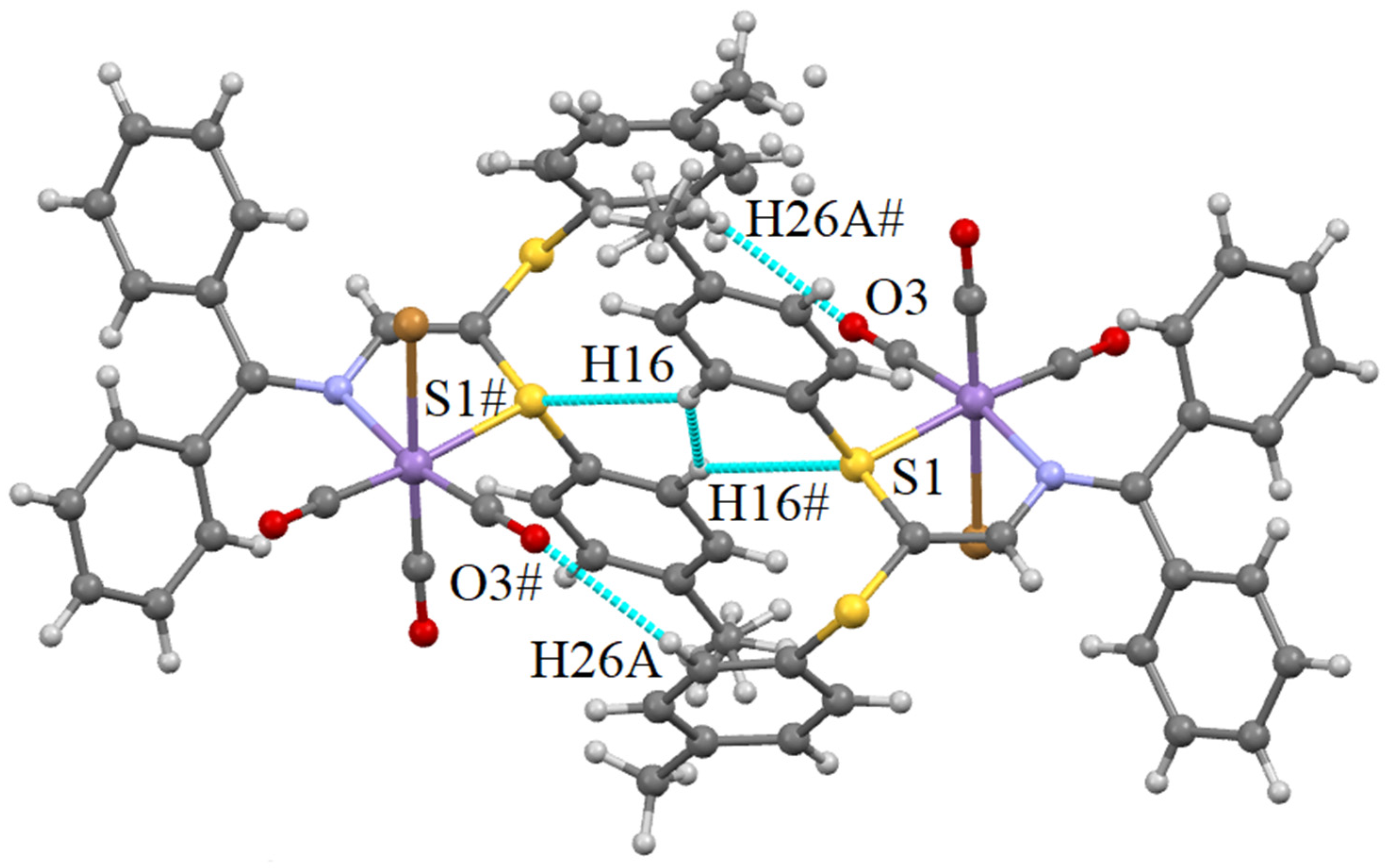
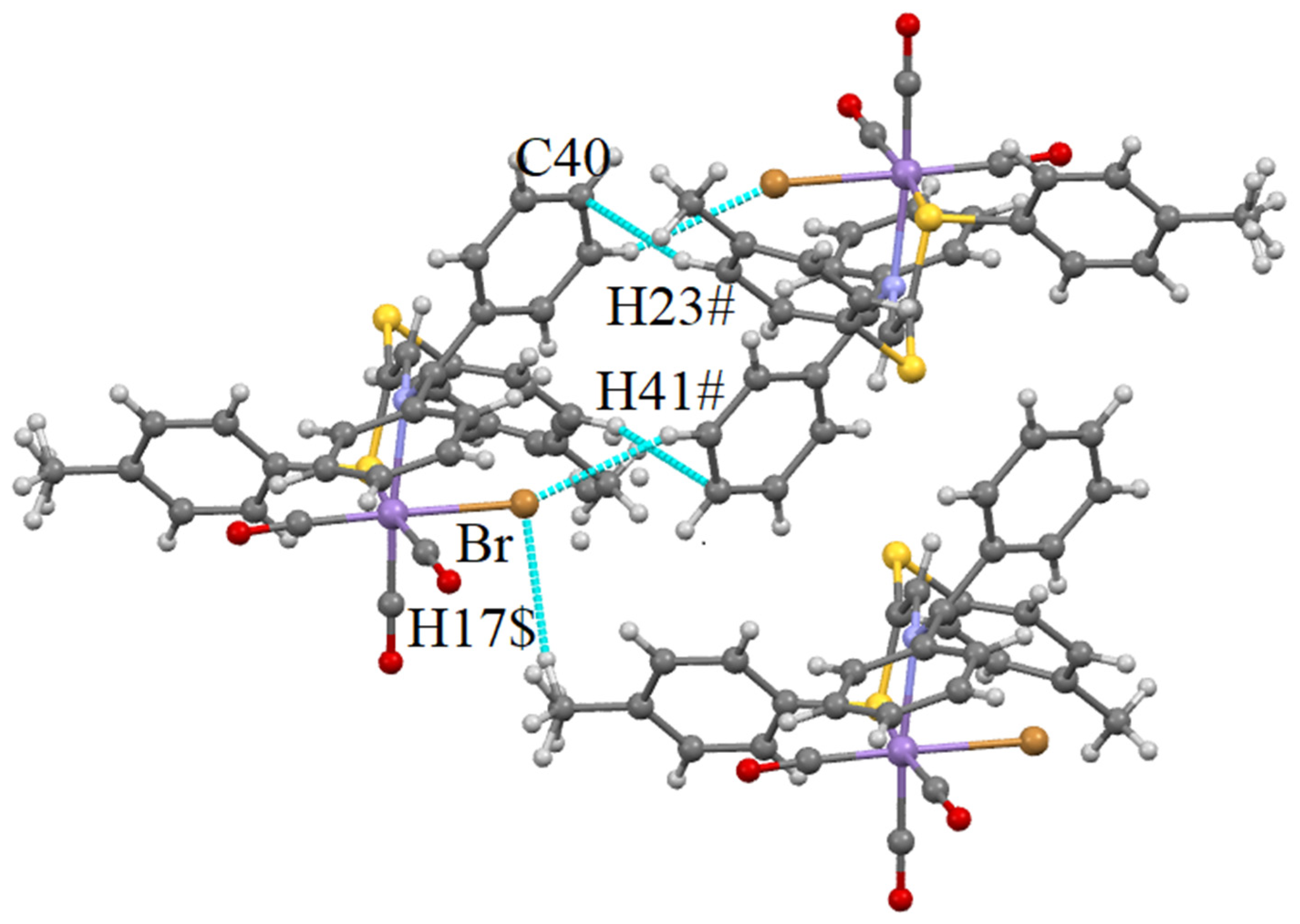
Supramolecular Features
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, M.J.; Scholz, D.; Tataruch, F.; Viehe, H.G. New Syntheses of Amino-Substituted 2-Azabutadienes. Chimia 1975, 29, 514–515. [Google Scholar] [CrossRef]
- Sato, N.; Adachi, J. Studies on pyrazines. 2. Structural assignment of the reaction of alpha-amino-alpha-phenylacetonitrile with chloral or bromal to N-(2,2-dihaloethenyl)-1-imino-1-phenylacetonitriles. J. Org. Chem. 1978, 43, 340–341. [Google Scholar] [CrossRef]
- Macicek, J.; Angelova, O.; Dryanska, V. Structures of 1,1-diphenyl-2-aza-1,3-butadienes. II. 3-Cyano-4-(n-fluorophenyl)-1,1-diphenyl-2-aza-1,3-butadienes (n = 2, 4). Acta Cryst. 1993, C49, 1818–1821. [Google Scholar] [CrossRef]
- King, R.B.; Hodges, K.C. Keteneimmonium and 2-Azabutadiene Complexes from Reactions of α-Chloroenamines with Metal Carbonyl Anions. J. Am. Chem. Soc. 1974, 96, 1263–1264. [Google Scholar] [CrossRef]
- Liu, F.; Yu, Y.; Liebeskind, L.S. N-Substituted Imines by the Copper-Catalyzed N-Imination of Boronic Acids and Organostannanes with O-Acyl Ketoximes. Org. Lett. 2007, 9, 1947–1950. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tarraga, A.; Molina, P. A Selective Chromogenic and Fluorescent Molecular Probe for YbIII Based on a Bichromophoric Azadiene. Europ. J. Inorg. Chem. 2010, 11, 697–703. [Google Scholar] [CrossRef]
- Jiang, N.J.; Melosso, M.; Bizzocchi, L.; Alessandrini, S.; Guillemin, J.-C.; Dore, L.; Puzzarini, C. Spectroscopic and Computational Characterization of 2-Aza-1,3-butadiene, a Molecule of Astrochemical Significance. Phys. Chem. A 2022, 126, 1881–1888. [Google Scholar] [CrossRef]
- Jacquot, S.; Belaissaoui, A.; Schmitt, G.; Laude, B.; Kubicki, M.M.; Blacque, O. Reaction of diphenyldiazomethane with N-methyloxy- and N-ethyloxycarbonyl-N-(2,2,2-trichloroethylidene)amines. Eur. J. Org. Chem. 1999, 1999, 1541–1544. [Google Scholar] [CrossRef]
- Jacquot-Rousseau, S.; Schmitt, G.; Khatyr, A.; Knorr, M.; Kubicki, M.M.; Vigier, E.; Blacque, O. Reactivity of 4,4-Dichloro-1,1-diphenyl-2-azabutadiene Towards Alkoxides and Thiolates: Synthesis of Functionalised π-Conjugated 2-Azabutadienes and Unexpected 1,4-Thiazine Formation. Eur. J. Org. Chem. 2006, 2006, 1555–1562. [Google Scholar] [CrossRef]
- Kinghat, R.; Boudiba, H.; Khatyr, A.; Knorr, M.; Kubicki, M.M. 4,4-Bis(4-methylphenylsulfanyl)-1,1-diphenyl-2-azabuta-1,3-diene. Acta Cryst. 2008, E64, o370. [Google Scholar] [CrossRef]
- Kinghat, R.; Schmitt, G.; Ciamala, K.; Khatyr, A.; Knorr, M.; Jacquot-Rousseau, S.; Rousselin, Y.; Kubicki, M.M. 1,3-Dipolar cycloaddition of diaryldiazomethanes across N-ethoxy-carbonyl-N-(2,2,2-trichloroethylidene)amine and reactivity of the resulting 2-azabutadienes towards thiolates and cyclic amides. Comptes Rendus Chim. 2016, 19, 320–332. [Google Scholar] [CrossRef]
- Kinghat, R.; Khatyr, A.; Knorr, M.; Strohmann, C.; Kubicki, M.M. 4,4-Bis(isopropylthio)-1,1-diphenyl-2-azabuta-1,3-diene Adducts with Cadmium(II), Mercury(II) and Copper(I) Iodides. Crystal, Molecular and Electronic Structures of d10 Transition Metal Chelate Complexes. Chemistry 2024, 6, 62–80. [Google Scholar] [CrossRef]
- Schlachter, A.; Juvenal, F.; Kinghat Tangou, R.; Khatyr, A.; Guyon, F.; Karsenti, P.L.; Strohmann, C.; Kubicki, M.M.; Rousselin, Y.; Harvey, P.D.; et al. 2-Azabutadiene complexes of rhenium(I): S,N-chelated species with photophysical properties heavily governed by the ligand hidden traits. Dalton Trans. 2021, 50, 2945–2963. [Google Scholar] [CrossRef]
- Mansour, A.M.; Radacki, K.; Phukan, H.J.; Roy, M.; Kumar, S.; Purkayastha, S.; Guha, A.K.; Srimani, D. Phototriggered cytotoxic properties of tricarbonyl manganese(I) complexes bearing α-diimine ligands towards HepG2. J. Biolog. Inorg. Chem. 2021, 26, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 5574–5584. [Google Scholar] [CrossRef]
- Amorim, A.L.; Peterle, M.M.; Guerreiro, A.; Coimbra, D.F.; Heying, R.S.; Caramori, G.F.; Braga, A.L.; Bortoluzzi, A.J.; Neves, A.; Bernardes, G.J.L.; et al. Synthesis, characterization and biological evaluation of new manganese metal carbonyl compounds that contain sulfur and selenium ligands as a promising new class of CORMs. Dalton Trans. 2019, 48, 5574–5584. [Google Scholar] [CrossRef] [PubMed]
- Skelton, B.W.; Tolhurst, V.-A.; White, A.H.; Williams, A.M.; Wilson, A.J. Synthesis and spectroscopic studies of organometallic Mn(I) complexes containing the novel mixed donor ligands 2 {MeSeCH(2-n)(SiMe3)n}C5H4N(n = 0-2). J. Organomet. Chem. 2003, 674, 38–44. [Google Scholar] [CrossRef]
- Mondal, A.; Pal, D.; Phukan, H.J.; Roy, M.; Kumar, S.; Purkayastha, S.; Guha, A.K.; Srimani, D. Manganese Complex Catalyzed Sequential Multi-component Reaction: Enroute to a Quinoline-Derived Azafluorenes. ChemSusChem 2024, 17, e202301138. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, S.E.A.; Durgaprasad, G.; Muthiah, K.A.T.; Rose, M.J. Tuning coordination modes of pyridine/thioether Schiff base (NNS) ligands to mononuclear manganese carbonyls. Dalton Trans. 2014, 43, 10725–10738. [Google Scholar] [CrossRef]
- Al-Masri, H.T.; Almejled, A.A.; Moussa, Z. Synthesis, characterization, X-ray structures, and catalytic activity of new Mn(I) and Re(I) metal complexes of chelating phosphinopyridylamine and its sulfide ligands. J. Organomet. Chem. 2025, 1032, 123620. [Google Scholar] [CrossRef]
- Gerber, T.I.A.; Betz, R.; Booysen, I.N.; Potgieter, K.C.; Mayer, P. Coordination of bidentate aniline derivatives to the fac-[Re(CO)3] + core. Polyhedron 2011, 30, 1739–1745. [Google Scholar] [CrossRef]
- Kinghat, R.; Khatyr, A.; Schmitt, G.; Knorr, M.; Kubicki, M.M.; Vigier, E.; Villafañe, F. Mono- and di-nuclear 2,3-diazabutadiene and 2-azabutadiene complexes of Rhenium(I): Syntheses, luminescence spectra and X-ray structures. Inorg. Chem. Commun. 2008, 11, 1060–1063. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P. Atoms in Molecules, An Introduction; Prentice Hall: London, UK, 2000. [Google Scholar]
- King, R.B. Organometallic Syntheses. Vol.1 Transition-Metal Compounds; Academic Press: New York, NY, USA, 1965; ISBN 0-444-42607-8. [Google Scholar]
- Hooft, R.W.W. COLLECT; Nonius BV: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Cryst. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas-Kroll-Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A. AIMAll (Version 17.11.14), TK Gristmill Software; AIMAll: Overland Park, KS, USA, 2017; Available online: http://aim.tkgristmill.com (accessed on 28 June 2025).



| 1 | 2 | |
|---|---|---|
| Mn–Br | 2.5326(4) | 2.5289(4) |
| Mn–S | 2.3454(7) | 2.3227(6) |
| Mn–N | 2.114(2) | 2.1565(17) |
| Mn-CBr | 1.801(3) | 1.793(2) |
| Mn–CS | 1.816(3) | 1.828(2) |
| Mn-CN | 1.808(3) | 1.793(2) |
| N–C1 | 1.312(3) | 1.321(3) |
| N–C2 | 1.407(3) | 1.408(3) |
| C2-C3 | 1.342(4) | 1.333(3) |
| S–Mn–N | 83.68(6) | 83.43(5) |
| Br–Mn–CBr | 178.11(9) | 178.06(7) |
| Compound | 1 | 2 |
|---|---|---|
| Formula | C30H21BrMnNO3S2 × CH2Cl2 | C32H25BrMnNO3S2 |
| Formula weight | 727.37 | 670.50 |
| Temperature/K | 115(2) | 115(2) |
| Wavelength/Å | 0.71073 | 0.71073 |
| Crystal system | orthorhombic | triclinic |
| Space group | P212121 | P |
| a/Å | 11.7945(3) | 10.7043(3) |
| b/Å | 14.1405(4) | 10.7338(4) |
| c/Å | 18.6642(5) | 13.4189(4) |
| a/° | 90 | 80.9070(10) |
| β/° | 90 | 77.926(2) |
| γ/° | 90 | 85.699(2) |
| Volume/Å3 | 3112.82(14) | 1487.34(8) |
| Z | 4 | 2 |
| ρ(calc.) g/cm3 | 1.552 | 1.497 |
| μ/mm−1 | 2.047 | 1.961 |
| F(000) | 1464 | 680 |
| Crystal size/mm | 0.18 × 0.11 × 0.11 | 0.20 × 0.10 × 0.10 |
| θ range for data collection/° | 1.81 to 27.47 | 1.57 to 27.46 |
| Index ranges | −15 ≤ h ≤ 15 −18 ≤ k ≤ 18 −24 ≤ l ≤ 24 | −13 ≤ h ≤ 13 −13 ≤ k ≤ 13 −17 ≤ l ≤ 17 |
| Reflections collected | 6761 | 12,170 |
| Independent reflections | 6761 | 6742 [R(int) = 0.0200] |
| Refl. greater [I > 2σ(I)] | 6523 | 6057 |
| Absorption correction | none | none |
| Transmission max | 0.4419 | 0.7980 |
| Transmission min | 0.3386 | 0.6494 |
| Refinement method | Full-matrix least squares on F2 | Full-matrix least squares on F2 |
| Data/restraints/parameters | 6761/0/372 | 6742/0/388 |
| Goodness-of-fit on F2 | 1.138 | 1.101 |
| Flack parameter | 0.045(6) | - |
| Final R indexes [I > 2σ(I)] | R1 = 0.0277 wR2 = 0.0580 | R1 = 0.0300 wR2 = 0.0612 |
| R indexes (all data) | R1 = 0.0306 wR2 = 0.0603 | R1 = 0.0370 wR2 = 0.653 |
| Largest diff. peak and hole/e. Å−3 | 0.293 and −0.306 | 0.399 and−0.346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinghat, R.; Khatyr, A.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Synthesis and Structural Characterization of Manganese(I) Complexes Ligated by 2-Azabutadienes (ArS)2C=C(H)-N=CPh2. Molbank 2025, 2025, M2042. https://doi.org/10.3390/M2042
Kinghat R, Khatyr A, Knorr M, Rousselin Y, Kubicki MM. Synthesis and Structural Characterization of Manganese(I) Complexes Ligated by 2-Azabutadienes (ArS)2C=C(H)-N=CPh2. Molbank. 2025; 2025(3):M2042. https://doi.org/10.3390/M2042
Chicago/Turabian StyleKinghat, Rodolphe, Abderrahim Khatyr, Michael Knorr, Yoann Rousselin, and Marek M. Kubicki. 2025. "Synthesis and Structural Characterization of Manganese(I) Complexes Ligated by 2-Azabutadienes (ArS)2C=C(H)-N=CPh2" Molbank 2025, no. 3: M2042. https://doi.org/10.3390/M2042
APA StyleKinghat, R., Khatyr, A., Knorr, M., Rousselin, Y., & Kubicki, M. M. (2025). Synthesis and Structural Characterization of Manganese(I) Complexes Ligated by 2-Azabutadienes (ArS)2C=C(H)-N=CPh2. Molbank, 2025(3), M2042. https://doi.org/10.3390/M2042







