Synthesis of a New Bichalcone via Suzuki–Miyaura Coupling Reaction
Abstract
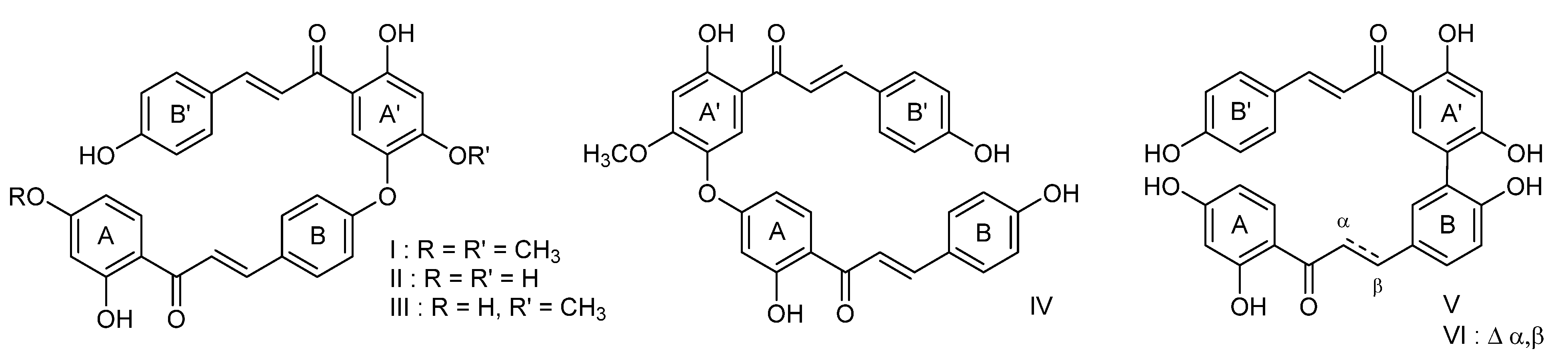
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Chemistry
3.2. Synthesis of 2-Bromo-3′,4,4′,5′-tetramethoxychalcone 5
3.3. Synthesis of 3-Borono-3′,4,4′,5′-tetramethoxychalcone 6
3.4. Synthesis of 3′,3‴,4,4′,4″,4‴,5′,5‴-Octamethoxy-2,3″-bichalcone 1
3.5. Synthesis of 4,4′-Dimethoxy-2,3′-bisbenzaldehyde 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-H.; Hsu, P.-H.; Hu, A.; Cheng, Y.-J.; Shih, T.-L.; Chen, J.-J. Synthesis of Flavonols and Assessment of Their Biological Activity as Anticancer Agents. Molecules 2024, 29, 2041. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Chaitanya, M.; Arunasree, K.M.; Alam, M.S. Synthesis of Some Novel Chalcones, Flavanones and Flavones and Evaluation of Their Anti-Inflammatory Activity. Eur. J. Med. Chem. 2013, 65, 51–59. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, Z.-N.; Zhang, J.-M.; Hou, X.-Y.; Yeh, S.M.; Ming, X. Recent Developments of Flavonoids with Various Activities. Curr. Top. Med. Chem. 2022, 22, 305–329. [Google Scholar] [CrossRef]
- Mbakidi-Ngouaby, H.; Pinault, E.; Gloaguen, V.; Costa, G.; Sol, V.; Millot, M.; Mambu, L. Profiling and Seasonal Variation of Chemical Constituents from Pseudotsuga menziesii Wood. Ind. Crops Prod. 2018, 117, 34–49. [Google Scholar] [CrossRef]
- Abdullah, I.; Phongpaichit, S.; Voravuthikunchai, S.P.; Mahabusarakam, W. Prenylated Biflavonoids from the Green Branches of Garcinia dulcis. Phytochem. Lett. 2018, 23, 176–179. [Google Scholar] [CrossRef]
- De Oliveira, J.C.S.; David, J.P.; David, J.M. Biflavonoids from the Bark Roots of Poincianella pyramidalis (Fabaceae). Phytochem. Lett. 2016, 16, 18–22. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Campos, V.R. Natural Biflavonoids as Potential Therapeutic Agents against Microbial Diseases. Sci. Total Environ. 2021, 769, 145168. [Google Scholar] [CrossRef]
- Ren, M.; Li, S.; Gao, Q.; Qiao, L.; Cao, Q.; Yang, Z.; Chen, C.; Jiang, Y.; Wang, G.; Fu, S. Advances in the Anti-Tumor Activity of Biflavonoids in Selaginella. Int. J. Mol. Sci. 2023, 24, 7731. [Google Scholar] [CrossRef]
- Nafisah, N.; Sugita, P.; Arifin, B.; Wahyudi, S.T. Biflavonoid Anti-Inflammatory Activity of the Araucariaceae Family—A Review. Trop. J. Phytochem. Pharm. Sci. 2024, 3, 411–423. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.A.; Fernández, W.A.; Trujillo, A.; Santos, J.C.; Roisnel, T.; Fuentealba, M. Synthesis, Characterization, and Crystal Structure of 2′,2″-Dihydroxy-3,3‴-Bichalcone and Its Related Chalcone–Flavanone and Biflavanone Analogs. Tetrahedron Lett. 2014, 55, 5271–5274. [Google Scholar] [CrossRef]
- Mdee, L.K.; Yeboah, S.O.; Abegaz, B.M. Rhuschalcones II-VI, Five New Bichalcones from the Root Bark of Rhus pyroides. J. Nat. Prod. 2003, 66, 599–604. [Google Scholar] [CrossRef]
- Li, H.-Y.; Nehira, T.; Hagiwara, M.; Harada, N. Total Synthesis and Absolute Stereochemistry of the Natural Atropisomer of the Biflavone 4′,4′′′,7,7′′-Tetra-O-Methylcupressuflavone. J. Org. Chem. 1997, 62, 7222–7227. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Flavin, M.T.; Cassidy, C.S.; Mar, A.; Chen, F.-C. Biflavonoids as Novel Antituberculosis Agents. Bioorganic Med. Chem. Lett. 2001, 11, 2101–2104. [Google Scholar] [CrossRef]
- Nising, C.F.; Schmid, U.K.; Nieger, M.; Bräse, S. A New Protocol for the One-Pot Synthesis of Symmetrical Biaryls. J. Org. Chem. 2004, 69, 6830–6833. [Google Scholar] [CrossRef]
- Mihigo, S.O.; Mammo, W.; Bezabih, M.; Andrae-Marobela, K.; Abegaz, B.M. Total Synthesis, Antiprotozoal and Cytotoxicity Activities of Rhuschalcone VI and Analogs. Bioorganic Med. Chem. 2010, 18, 2464–2473. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.D.; McGown, A.T.; Lawrence, N.J. Combretastatin-like Chalcones as Inhibitors of Microtubule Polymerization. Part 1: Synthesis and Biological Evaluation of Antivascular Activity. Bioorganic Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef]
- Boumendjel, A.; McLeer-Florin, A.; Champelovier, P.; Allegro, D.; Muhammad, D.; Souard, F.; Derouazi, M.; Peyrot, V.; Toussaint, B.; Boutonnat, J. A Novel Chalcone Derivative Which Acts as a Microtubule Depolymerising Agent and an Inhibitor of P-Gp and BCRP in in-Vitro and in-Vivo Glioblastoma Models. BMC Cancer 2009, 9, 242. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A Retrospective-Prospective Review of Suzuki–Miyaura Reaction: From Cross-Coupling Reaction to Pharmaceutical Industry Applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Hurtová, M.; Biedermann, D.; Osifová, Z.; Cvačka, J.; Valentová, K.; Křen, V. Preparation of Synthetic and Natural Derivatives of Flavonoids Using Suzuki–Miyaura Cross-Coupling Reaction. Molecules 2022, 27, 967. [Google Scholar] [CrossRef]
- Baltus, C.B.; Press, N.J.; Spencer, J. Microwave-Mediated Suzuki–Miyaura Cross-Couplings of Thioether- and Ortho-Substituted Methylphenylboronic Acid Esters. Synlett 2012, 23, 2477–2480. [Google Scholar] [CrossRef]
- Tan, J.; Gu, X.; Dai, H.; Song, Y.; Huang, Z.; Zhao, Y. A Nickel-Catalyzed Suzuki-Miyaura Coupling Reaction of Aryl Halides Facilitated by Pyridine Derivatives. Tetrahedron Lett. 2024, 143, 155130. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, L.; Li, J.; Xie, W. A General Copper Catalytic System for Suzuki–Miyaura Cross-Coupling of Unactivated Secondary and Primary Alkyl Halides with Arylborons. J. Am. Chem. Soc. 2023, 145, 28146–28155. [Google Scholar] [CrossRef]
- Crockett, M.P.; Tyrol, C.C.; Wong, A.S.; Li, B.; Byers, J.A. Iron-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions between Alkyl Halides and Unactivated Arylboronic Esters. Org. Lett. 2018, 20, 5233–5237. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, K.; Edler, M.C.; Hamel, E.; Mooberry, S.L.; Paige, M.A.; Brown, M.L. A Boronic Acid Chalcone Analog of Combretastatin A-4 as a Potent Anti-Proliferation Agent. Bioorganic Med. Chem. 2010, 18, 971–977. [Google Scholar] [CrossRef]
- Pouget, C.; Trouillas, P.; Gueye, R.; Champavier, Y.; Laurent, A.; Duroux, J.-L.; Sol, V.; Fagnere, C. Exploring the Use of the Suzuki Coupling Reaction in the Synthesis of 4′-Alkyl-2′-Hydroxyacetophenones. Synlett 2014, 25, 564–568. [Google Scholar] [CrossRef]

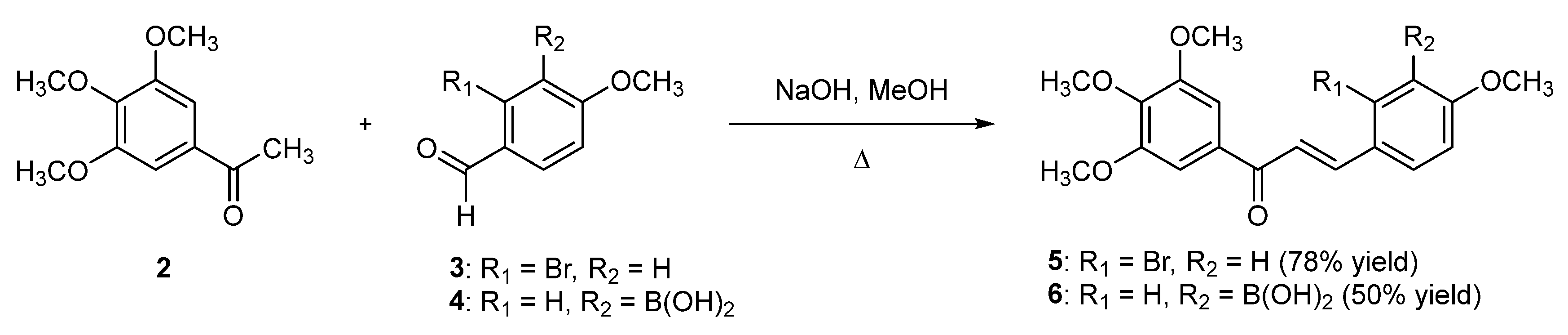


| Exp. | 4 | Catalyst | Time MW Heating | Yield |
|---|---|---|---|---|
| 1 | 1.1 eq | PdCl2-dppf 0.2 eq | 2 × 10 min | 30% |
| 2 | 1.4 eq | PdCl2-dppf 0.2 eq | 2 × 10 min | 38% |
| 3 | 1.4 eq | PdCl2-dppf 0.2 eq | 5 × 10 min | 48% |
| 4 | 2.0 eq | PdCl2-dppf 0.2 eq | 5 × 10 min | 35% |
| 5 | 1.4 eq | Pd(PPh3)4 0.2 eq | 5 × 10 min | 34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toublet, F.-X.; Champavier, Y.; Lévêque, A.; Fagnère, C.; Pouget, C. Synthesis of a New Bichalcone via Suzuki–Miyaura Coupling Reaction. Molbank 2025, 2025, M2012. https://doi.org/10.3390/M2012
Toublet F-X, Champavier Y, Lévêque A, Fagnère C, Pouget C. Synthesis of a New Bichalcone via Suzuki–Miyaura Coupling Reaction. Molbank. 2025; 2025(2):M2012. https://doi.org/10.3390/M2012
Chicago/Turabian StyleToublet, François-Xavier, Yves Champavier, Aurélie Lévêque, Catherine Fagnère, and Christelle Pouget. 2025. "Synthesis of a New Bichalcone via Suzuki–Miyaura Coupling Reaction" Molbank 2025, no. 2: M2012. https://doi.org/10.3390/M2012
APA StyleToublet, F.-X., Champavier, Y., Lévêque, A., Fagnère, C., & Pouget, C. (2025). Synthesis of a New Bichalcone via Suzuki–Miyaura Coupling Reaction. Molbank, 2025(2), M2012. https://doi.org/10.3390/M2012






