4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one)
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
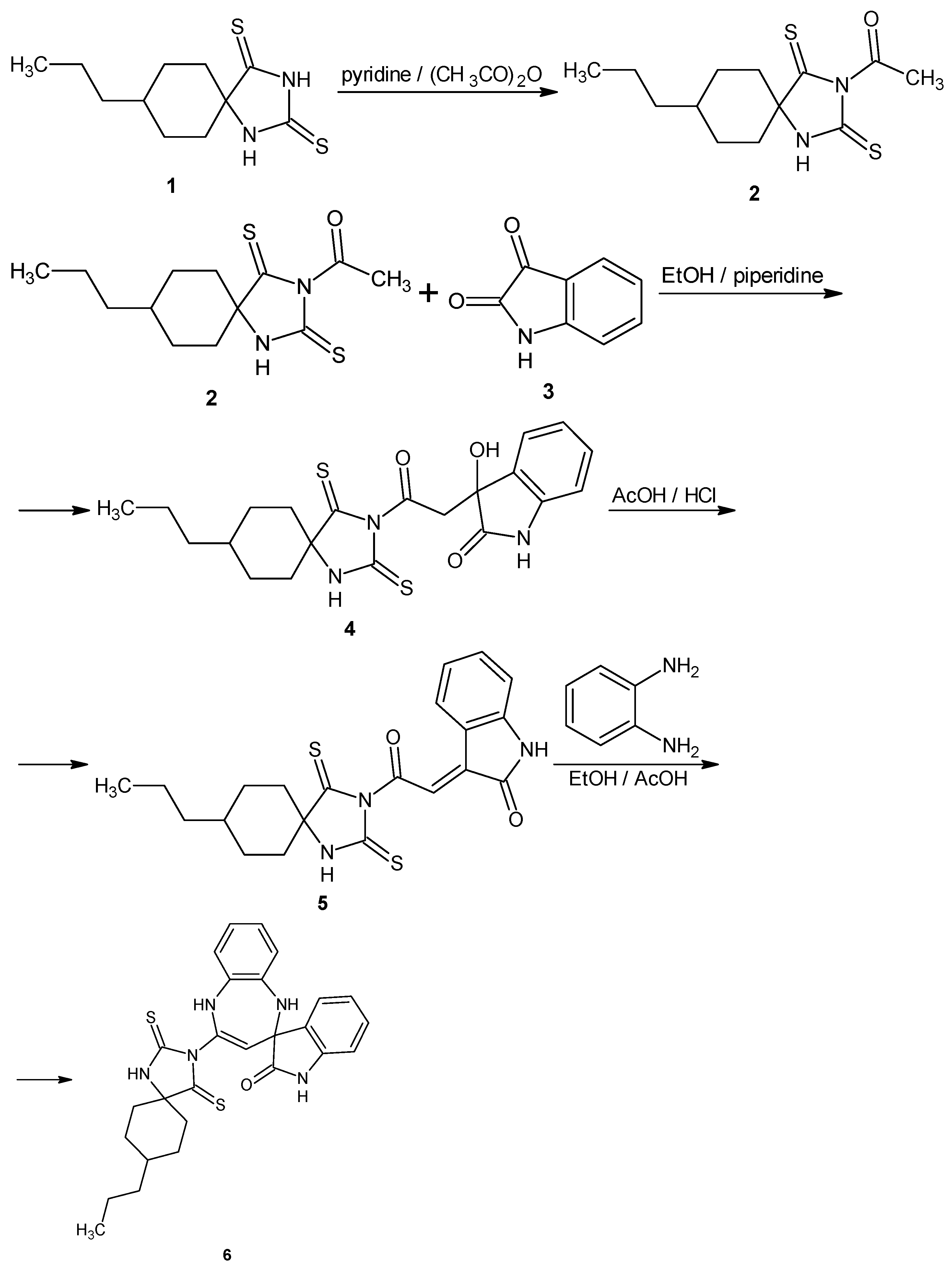
3.1. Synthesis
3.2. Synthesis of 3-Hydroxy-3-[2-oxo-2-(8-propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)ethyl]indolin-2-one (4)
3.3. Synthesis of 3-[2-Oxo-2-(8-propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)ethylidene]indolin-2-one (5)
3.4. Synthesis of 4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one (6)
3.5. Apparatus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grewal, A. Isatin Derivatives with Several Biological Activities. Int. J. Pharm. Res. 2014, 6, 1–7. [Google Scholar]
- Pakravan, P.; Kashanian, S.; Khodaei, M.; Harding, F. Biochemical and pharmacological characterization of isatin and its derivatives: From structure to activity. Pharmacol. Rep. 2013, 65, 313–335. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Hao, Z.; Zhang, X.; Wang, L. Efficient Synthesis and Biological Evaluation of a Novel Series of 1,5-Benzodiazepine Derivatives as Potential Antimicrobial Agents. Chem. Biol. Drug Des. 2016, 88, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Caiana, E.; de Veras, B.O.; de Souzaa, A.L.; Queiroz, N. Synthesis of Hydroxybenzodiazepines with Potential Antioxidant and Antifungal Action. J. Braz. Chem. Soc. 2021, 32, 626–637. [Google Scholar] [CrossRef]
- Elgazwy, A.S.; Atta-Allha, S.; Keshk, S. Synthesis of 5-spirocyclohexyl-2,4-dithiohydantoin derivatives: A potential anti-leishmaniasis agent. Monatsh. Chem. 2009, 140, 243–249. [Google Scholar] [CrossRef]
- Lloyd, D.; Cleghorn, H. 1,5-Benzodiazepines. Adv. Heterocycl. Chem. 1974, 17, 27–43. [Google Scholar] [CrossRef]
- Obydennov, D.; Sosnovskikh, V. The reaction of 6-substituted 4-pyrone-2-carboxylic acids with o-phenylenediamine. Synthesis and structure of 3-(1H-1,5-benzodiazepin-2(3H)-ylidenemethyl)quinoxalin-2(1H)-ones. Chem. Heterocycl. Compd. 2015, 51, 281–290. [Google Scholar] [CrossRef]
- Obydennov, D.; Sosnovskikh, V. 3-(1H-1,5-Benzodiazepin-2(3H)-ylidenemethyl)-quinoxalin-2(1H)-ones in reactions with nucleophiles. Synthesis and structure of 3-(hetarylmethyl)quinoxalin-2-ones. Chem. Heterocycl. Compd. 2015, 51, 503–513. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, Y. Synthesis, spectral studies and biological activity of 3H-1, 5-benzodiazepine derivatives. ARKIVOC 2007, xiii, 142–149. [Google Scholar] [CrossRef]
- Odame, F.; Madanhire, T.; Tettey, C.; Neglo, D.; Adzaho, F.; Sedohia, D.; Hosten, E. Anticancer and Antimicrobial Activity of Some New 2,3-Dihydro-1,5-benzodiazepine Derivatives. Heteroat. Chem. 2023, 2023, 3390122. [Google Scholar] [CrossRef]
- Jaafar, Z.; Chniti, S.; Sassi, A.; Dziri, H.; Marque, S.; Lecouvey, M.; Gharbi, R.; Msaddek, M. Design and microwave-assisted synthesis of dimers of 1,5 benzodiazepine-1,2,3-triazole hybrids bearing alkyl/aryl spacers and their biological assessment. J. Mol. Struct. 2019, 1195, 689–701. [Google Scholar] [CrossRef]
- Liu, J.B.; Lin, C.L.; Wan, Y.Q.; Song, H.C. Synthesis and Bioactivities of 1,5-Benzodiazepine Derivatives. Chin. J. Org. Chem. 2008, 28, 317–320. [Google Scholar]
- Wang, L.; Li, X.; An, Y. 1,5-Benzodiazepine derivatives as potential antimicrobial agents: Design, synthesis, biological evaluation, and structure–activity relationships. Org. Biomol. Chem. 2015, 13, 5497–5509. [Google Scholar] [CrossRef] [PubMed]
- Nsira, A.; Mtiraoui, H.; Chniti, S.; Al-Ghulikah, H.; Gharbi, R.; Msaddek, M. Regioselective One-Pot Synthesis, Biological Activity and Molecular Docking Studies of Novel Conjugates N-(p-Aryltriazolyl)-1,5-benzodiazepin-2-ones as Potent Antibacterial and Antifungal Agents. Molecules 2022, 27, 4015. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Bhatia, R.; Singh, G.; Kumar, B.; Mehan, S.; Monga, V. Design, Synthesis and Neuropharmacological Evaluation of New 2,4-Disbsituted-1,5 Benzodiazepines as CNS Active Agents. Bioorg. Chem. 2020, 101, 104010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Le, V.; Xu, X.; Shao, X.; Liu, J.; Li, Z. Discovery of novel 1,5-benzodiazepine-2,4-dionederivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 3948–3951. [Google Scholar] [CrossRef] [PubMed]
- Penchev, P.N.; Schulz, K.-P.; Munk, M.E. INFERCNMR: A 13C NMR Interpretive Library Search System. J. Chem. Inf. Model. 2012, 52, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDBConstructing a Free Chemical Information System with Open-Source Components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, C.; Kuhn, S. NMRShiftDB–compound identification and structure elucidation support through a free community-built web database. Phytochemistry 2004, 65, 2711–2717. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide, Recognition of Structural Fragments by NMR, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, p. 27. [Google Scholar]
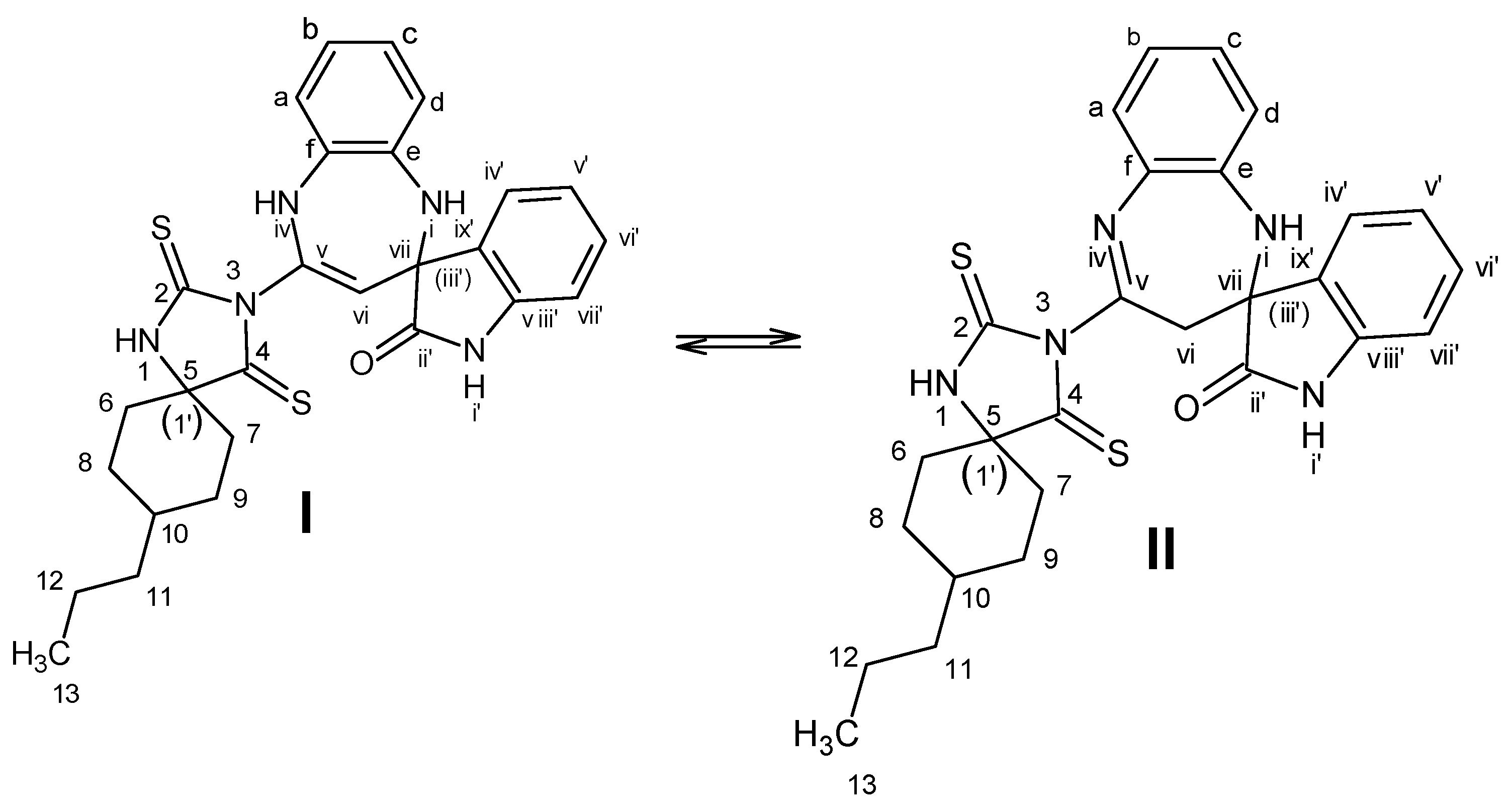
| Atom | δ (13C), ppm | DEPT b | δ (1H), ppm | Multiplicity (J, Hz) | 1H-1H COSY b | HMBC b |
|---|---|---|---|---|---|---|
| 2(C=S) | 180.25 | C | ||||
| 4(C=S) | 208.63 | C | ||||
| 1(NH) | 13.91 | s | ||||
| (1′) | 79.79 | C | ||||
| 6/7 | 30.77 | CH2 | 2.66 c (Ha) f | td(13.4, 4.7) | Hb, Hc, Hd | (1′), 8/9, 4 |
| 1.80 c (Hb) f | m | Ha, Hc, Hd | ||||
| 8/9 | 21.13 | CH2 | 2.20 c (Hc) f | qd(13.2, 4.0) | Hd, Ha, Hb, 10 | 6/7 d, 10 e |
| 1.59 c (Hd) f | m | Hc, Ha, Hb, 10 | ||||
| 10 | 45.05 | CH | 1.06 c | m | Hc, Hd | |
| 11 | 20.31 | CH2 | 2.17 c (He) f | qd(13.2, 4.0) | Hf, 10, Hg, Hh | |
| 1.55 c (Hf) f | m | He, 10, Hh, Hg | ||||
| 12 | 35.05 | CH2 | 1.86 c (Hg) f | m | He, Hf, Hh | |
| 1.63 c (Hh) f | m | He, Hf, Hg | ||||
| 13 | 27.38 | CH3 | 0.86 c | s | 10 | |
| ii′(C=O) | 176.15 | C | ||||
| v | 171.30 | C | ||||
| i(NH) | 7.26 | s | ||||
| (iii′) | 74.30 | C | ||||
| iv(NH) | ||||||
| i′(NH) | 10.34 | s | (iii′), viii′, ix′ | |||
| viii′ | 141.30 | C | ||||
| ix′ | 129.78 | C | ||||
| iv′ | 125.59 | CH | 7.55 | d(7.4) | v′, vi′ e | (iii′), viii′, vi′ |
| v′ | 122.44 | CH | 7.07 c | td(7.6, 0.9) | iv′, vi′, vii′ e | vii′, ix′ |
| vi′ | 130.46 | CH | 7.29 | td(7.7, 1.2) | v′, vii′, iv′ e | iv′, viii′ |
| vii′ | 110.03 | CH | 6.84 | d(7.8) | v′ e, vi′ | v′, ix′ |
| vi | 29.17/32.14 | CH/CH2 | 2.72 | s | v | |
| a | 121.07 | CH | 7.04 c,f | dd(7.5,0.8) | b, c e | c, e |
| b | 118.63 | CH | 6.63 f | td(7.5, 1.0) | a, c, d e | d, f |
| c | 125.68 | CH | 6.90 f | td(7.7, 1.2) | a e, b, d | a, e |
| d | 108.41 | CH | 6.53 f | dd(7.8, 0.8) | b e, c | b, f |
| e | 147.19 | C | ||||
| f | 124.10 | C |
| ATR-IR Band | Wavenumber, cm−1 |
|---|---|
| v(N-H) | 3313 |
| v(C-H) | 3136, 3085, 3070, 3060 |
| vas(CH3) | 2953 |
| vas(CH2) | 2937 |
| v(C=O) | 1705 |
| v(C=C) | 1603, 1493 |
| δs(CH2) | 1473 |
| v(C=S) | 1257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoitsov, D.; Marinov, M.; Penchev, P.; Stoyanov, N. 4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one). Molbank 2025, 2025, M2011. https://doi.org/10.3390/M2011
Stoitsov D, Marinov M, Penchev P, Stoyanov N. 4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one). Molbank. 2025; 2025(2):M2011. https://doi.org/10.3390/M2011
Chicago/Turabian StyleStoitsov, Dimitar, Marin Marinov, Plamen Penchev, and Neyko Stoyanov. 2025. "4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one)" Molbank 2025, no. 2: M2011. https://doi.org/10.3390/M2011
APA StyleStoitsov, D., Marinov, M., Penchev, P., & Stoyanov, N. (2025). 4-(8-Propyl-2,4-dithioxo-1,3-diazaspiro[4.5]decan-3-yl)spiro[1,5-dihydro-1,5-benzodiazepine-2,3′-indoline]-2′-one). Molbank, 2025(2), M2011. https://doi.org/10.3390/M2011






