Synthesis of Mesoionic 1,3,4-Thiadiazole-2-Thiolates
Abstract
1. Introduction
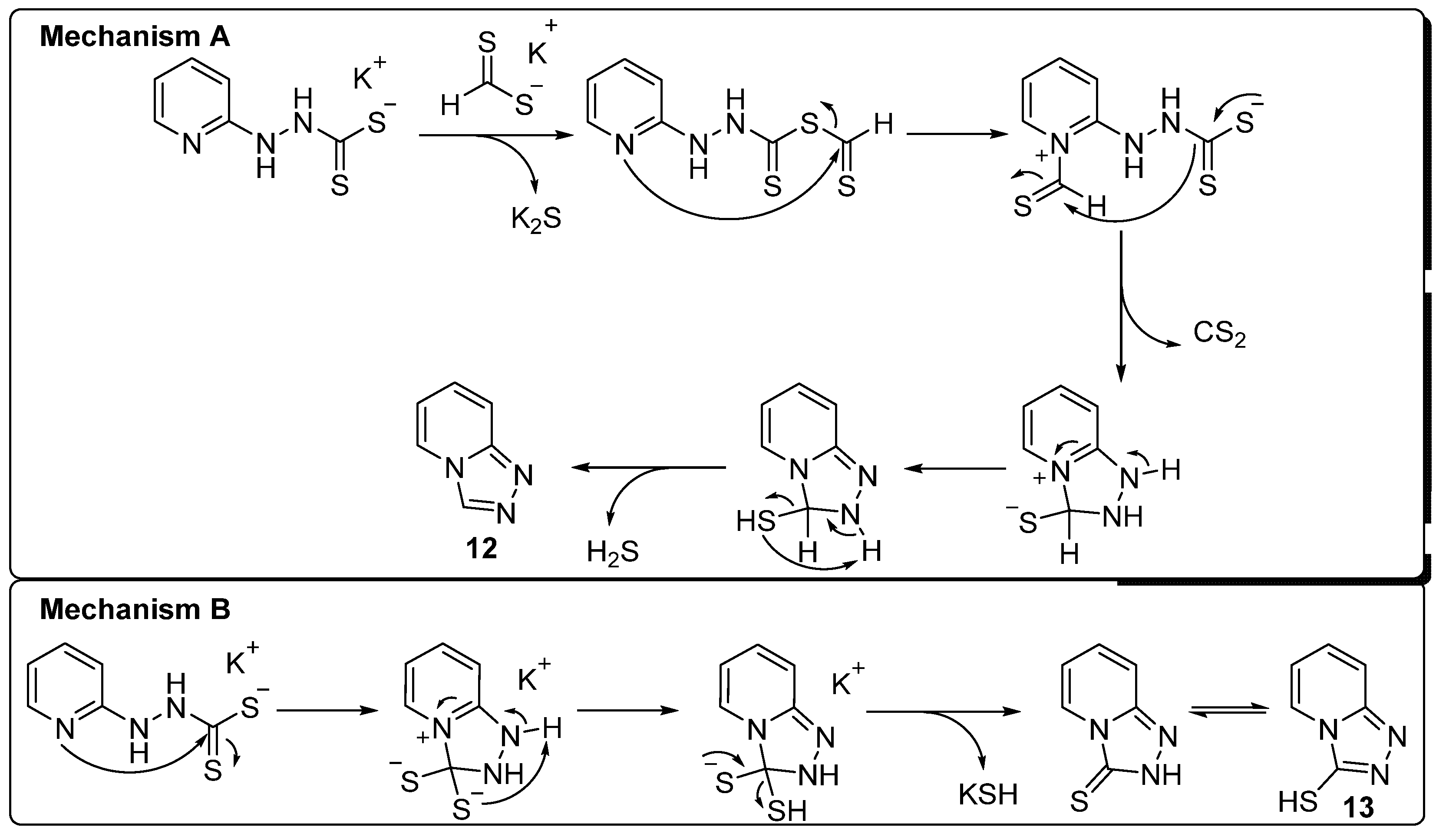
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Information
3.2. Experimental Procedures and Characterization of Compounds
- (1)
- General procedure for the conversion of hydrazine hydrochlorides to free hydrazines
- (2)
- General procedure for the synthesis of potassium hydrazinecarbodithioates:
- (3)
- General procedure for the synthesis of 1,3,4-thiadiazolium-2-thiolates:
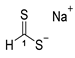
3.2.1. Sodium Dithioformate (Sodium Methanedithioate) (11a)
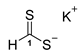
3.2.2. Potassium Dithioformate (Potassium Methanedithioate) (11b)
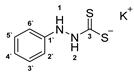
3.2.3. Potassium Phenylhydrazinecarbodithioate (10a)
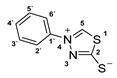
3.2.4. 4-Phenyl-1,3,4-Thiadiazolium-2-Thiolate (1a)
3.2.5. Potassium 1-(4′-Chlorophenyl)Hydrazinecarbodithioate (10b)
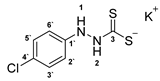
3.2.6. 4-(4′-Chlorophenyl)-1,3,4-Thiadiazolium-2-Thiolate (1b)
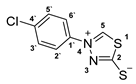
3.2.7. Potassium 1-(P-Tolyl)Hydrazinecarbodithioate (10c)
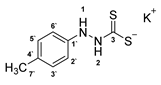
3.2.8. 4-(P-Tolyl)-1,3,4-Thiadiazolium-2-Thiolate (1c)
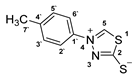
3.2.9. Potassium 1-(4′-Methoxyphenyl)Hydrazinecarbodithioate (10d)
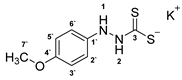
3.2.10. 4-(4′-Methoxyphenyl)-1,3,4-Thiadiazolium-2-Thiolate (1d)
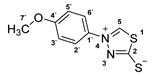
3.2.11. Potassium 1-(Pyridine-2-Yl)Hydrazinecarbodithioate (10e)
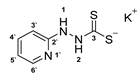
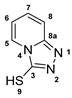
3.2.12. [1,2,4]Triazolo[4,3-a]Pyridine (12)
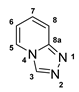
3.2.13. [1,2,4]Triazolo[4,3-a]Pyridine-3-Thiol (13)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Busch, M. Synthese von Biazolinderivaten. Ber. Dtsch. Chem. Ges. 1895, 28, 2635–2647. [Google Scholar] [CrossRef]
- Ollis, W.D.; Ramsden, C.A. Mesoionic compounds. Adv. Heterocycl. Chem. 2022, 137, 229–347. [Google Scholar]
- Ollis, W.D.; Stanforth, S.P.; Ramsden, C.A. Heterocyclic mesomeric betaines. Tetrahedron 1985, 41, 2239–2329. [Google Scholar] [CrossRef]
- Fischer, E. Ueber die Hydrazinverbindungen. Justus Liebigs Ann. Chem. 1882, 212, 316. [Google Scholar] [CrossRef]
- Kushi, Y.; Fernando, Q. Crystal and molecular structure of the meso-ionic sydnone, anhydro-5-mercapto-2,3-diphenyltetrazolium hydroxide. J. Am. Chem. Soc. 1970, 92, 1965–1968. [Google Scholar] [CrossRef]
- Khan, T.; Yadav, R.; Kesharwani, A.K.; Chourasia, K. A review on synthesis, characterization, and pharmacological properties of some sydnone derivatives. Mini-Rev. Org. Chem. 2025, 22, 359–380. [Google Scholar] [CrossRef]
- Zerbib, S.; Khouili, M.; Catto, M.; Bouissane, L. Sydnone: Synthesis, reactivity and biological activities. Curr. Med. Chem. 2023, 30, 1122–1144. [Google Scholar] [CrossRef]
- Cherepanov, I.; Moiseev, S.K. Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar]
- Freese, T.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sulfur, mercury, and boron adducts of sydnone imine derived anionic N-heterocyclic carbenes. RSC Adv. 2019, 9, 4781–4788. [Google Scholar] [CrossRef]
- Mummel, S.; Lederle, F.; Hübner, E.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sydnone methides—A forgotten class of mesoionic compounds for the generation of anionic N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2021, 60, 18882–18887. [Google Scholar] [CrossRef]
- Reissig, H.-U. Münchnones—New factes after 50 years. Angew. Chem. Int. Ed. 2014, 53, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Beall, L.S.; Heidelbaugh, T.M.; Liu, B.; Sheehan, S.M. A One-pot bicycloannulation method for the synthesis of tetrahydroisoquinoline systems. J. Org. Chem. 2000, 65, 2684–2695. [Google Scholar] [CrossRef]
- Nein, Y.I.; Morzherin, Y.Y. Criteria for aromaticity of mesoionic heterocycles. Russ. Chem. Bull. 2012, 61, 1111–1116. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Oziminski, W.P. A quantitative analysis of factors influencing ease of formation and σ-bonding strength of oxa- and thia-n-heterocyclic carbenes. J. Org. Chem. 2017, 82, 12485–12491. [Google Scholar] [CrossRef]
- Mitsubishi Paper Mills, Ltd. Japan. Developers Containing 3-Thioxo-1,2-diazole Inner Salts for Lithographic Plate Making by Silver Complex Diffusion-Transfer Process. JP04328559 A, 17 November 1992. [Google Scholar]
- Irving, H.M.N.H.; Kiwan, A.M. Effect of solvent on the electronic absorption spectra of the mesoionic compounds 2,3-diphenyl-2H-tetrazolium-5-thiolate and 4-phenyl-1,3,4-thiadiazolium-2-thiolate. J. Chem. Soc. B 1971, 5, 898–901. [Google Scholar]
- Ollis, W.D.; Ramsden, C.A. Cyclic mesoionic compounds. XIII. Mass spectra of mesoionic heterocycles. J. Chem. Soc. Perkin Trans. 1 1974, 6, 645–650. [Google Scholar] [CrossRef]
- Atkin, C.W.; Barnes, A.N.M.; Edgerley, P.G.; Sutton, L.E. Cyclic mesoionic compounds. VII. Electric dipole moments of some mesoionic 1,3,4-thiadiazoles. J. Chem. Soc. B 1969, 9, 1194–1196. [Google Scholar] [CrossRef]
- Baker, W.; Ollis, W.D. Meso-ionic compounds. Quart. Rev. 1957, 11, 15–29. [Google Scholar] [CrossRef]
- Kier, L.B.; Roche, E.B. Medicinal chemistry of the mesoionic compounds. J. Pharmaceut. Sci. 1967, 56, 149–168. [Google Scholar] [CrossRef]
- Ohta, N.; Kato, H.; Kaneko, T. Structure of busch’s endo-thiatriazolines. Bull. Chem. Soc. Jap. 1967, 40, 579–583. [Google Scholar] [CrossRef]
- Grashey, R.; Baumann, M.; Lubos, W.-D. Mesoionische 1,3,4-Thiadiazol-2-thione. Tetrahedron Lett. 1968, 56, 5881–5884. [Google Scholar] [CrossRef]
- Kier, L.B.; Scott, M.K. The synthesis of dialkyl mesoionic 1,3,4-thiadiazoles. J. Heterocycl. Chem. 1968, 5, 277–279. [Google Scholar] [CrossRef]
- Levi, T.G. Dithioformic acid II. Atti Accad. Naz. Lincei 1929, 9, 170–175. [Google Scholar]
- Gattow, G.; Dräger, M.; Engler, R. Über Dithioformiate. Naturwissenschaften 1971, 58, 53. [Google Scholar]
- Engler, R.; Gattow, G.; Dräger, M. Untersuchungen über Thioameisensäuren. 2. Darstellung und Eigenschaften von Dithioformiaten. Z. Anorg. Allg. Chem. 1972, 388, 229–237. [Google Scholar] [CrossRef]
- Martin, K. Potassium dithioformiate synthesis. Chem. Br. 1988, 24, 427–428. [Google Scholar]
- Urben, P.G.; Pitt, M.J. (Eds.) . Bretherick’s Handbook of Reactive Chemical Hazards, 6th ed.; Butterworth Heinemann: Oxford, UK, 1999; Volume 1, pp. 145–146. [Google Scholar]
- Muraoka, M.; Yamamoto, T.; Enomoto, K.; Takeshima, T. Synthesis of 2-alkoxycarbonyl enamino thioaldehydes and selenaldehydes (as pentacarbonyltungsten (0) complexes). Improved synthesis of simple and 2-cyano enamino thioaldehydes and some chemical reactions of these compounds. J. Chem. Soc. Perkin Trans. 1 1989, 7, 1241–1252. [Google Scholar] [CrossRef]
- Schauer, S.J.; Eyman, D.P.; Bernhardt, R.J.; Wolff, M.A.; Mallis, L.M. Synthesis and reactivity of (ŋ6-C6H6)Mn(CO)2SC(S)H and (ŋ6-C6(CH3)6)Mn(CO)2SC(S)H. Inorg. Chem. 1991, 30, 570–572. [Google Scholar] [CrossRef]
- Binder, H.; Diamantikos, W. Die Reaktion zwischen Schwefel bzw. Kohlenstoflfdisulfid und Natriumboranat in Aminen. Zwei neue Darstellungsmethoden für Alkylaminborane, R3-nHnN-BH3. Z. Naturforsch. 1983, 38b, 203–207. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, J.; Xin, M.; Jiang, Y.; Du, Z.; Lu, G.; Jiang, J.; Chan, A.S.C.; Ke, Z.; Zou, Y. Chalcone-based synthesis of tetrahydropyridazines via cloke–wilson-type rearrangement-involved tandem reaction between cyclopropyl ketones and hydrazines. J. Org. Chem. 2024, 89, 2726–2740. [Google Scholar] [CrossRef]
- Baker, W.; Ollis, W.D.; Phillips, A.; Strawford, T. Cyclic meso-ionic compounds. Part IV. ψ-4-Aryl-2: 4-dihydro-2-thio-1-thia-3: 4-diazoles (“endothiodihydrothiodiazoles”). J. Chem. Soc. 1951, 0, 289–291. [Google Scholar] [CrossRef]
- Guo, H.M.; Wang, J.J.; Xiong, Y.; Wu, X. Visible-light-driven multicomponent reactions for the versatile synthesis of thioamides by radical thiocarbamoylation. Angew. Chem. Int. Ed. 2024, 63, e202409605. [Google Scholar] [CrossRef]
- Li, E.; Hu, Z.; Song, L.; Yu, W.; Chang, J. Synthesis of 1,2,4-triazolo[4,3-a]pyridines and related heterocycles by sequential condensation and iodine-mediated oxidative cyclization. Chem. Eur. J. 2016, 22, 11022–11027. [Google Scholar] [CrossRef]
- Fargher, R.G.; Furness, R. Derivatives of 2-pyridylhydrazine and 2-quinolylhydrazine. J. Chem. Soc. Perkin Trans. 1 1915, 107, 688–699. [Google Scholar] [CrossRef]
- Tarbell, D.S.; Todd, C.W.; Paulson, M.C.; Lindstrom, E.G.; Wystrach, V.P. Synthesis of some substituted thiocarbazones. J. Am. Chem. Soc. 1948, 70, 1381–1385. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Ahamedzade, M.; Kirilmis, C.; Cukurovali, A.; Dilsiz, N. Synthesis and antimicrobial activity of new thiazole-2H(3H)-thiones containing 1,1,3-trisubstituted cyclobutene. S. Afr. J. Chem. 2003, 56, 21–24. [Google Scholar]
- Cambi, L.; Bargigia, G.; Paglia, E.D.; Ricca, G.S. Sui prodotti di riduzione dei tetrazo-tritioderivati dagli acidi ditiocarbazici. Atti Della Accad. Naz. Dei Lincei. Cl. Di Sci. Fis. Mat. E Naturali. Rend. 1968, 45, 330–338. [Google Scholar]
- Busch, M. Über heterobicyklische Verbindungen der Thiobiazol- und Triazolreihe. J. prakt. Chem. 1903, 67, 201–215. [Google Scholar] [CrossRef]
- Cmoch, P.; Stefaniak, L.; Melzer, E.; Bałoniak, S.; Webb, G.A. 1H, 13C and 15N NMR study of some triazolo- and tetrazolopyridazines and thioxotriazolopyridines. Magn. Reson. Chem. 1999, 37, 493–497. [Google Scholar] [CrossRef]


| Compound Number | Compound Structure | Yield [%] |
|---|---|---|
| 10a | 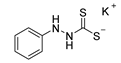 | 91 |
| 10b | 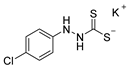 | 92 |
| 10c |  | 84 |
| 10d |  | 74 |
| 10e | 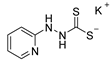 | 66 |
| Compound Number | Compound Structure | Yield [%] |
|---|---|---|
| 1a |  | 69 |
| 1b | 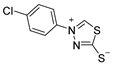 | 60 |
| 1c | 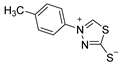 | 71 |
| 1d | 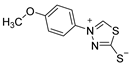 | 65 |
| 1e | 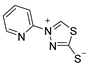 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahnert, S.R.; Schmidt, A. Synthesis of Mesoionic 1,3,4-Thiadiazole-2-Thiolates. Molbank 2025, 2025, M2010. https://doi.org/10.3390/M2010
Kahnert SR, Schmidt A. Synthesis of Mesoionic 1,3,4-Thiadiazole-2-Thiolates. Molbank. 2025; 2025(2):M2010. https://doi.org/10.3390/M2010
Chicago/Turabian StyleKahnert, Sean Ray, and Andreas Schmidt. 2025. "Synthesis of Mesoionic 1,3,4-Thiadiazole-2-Thiolates" Molbank 2025, no. 2: M2010. https://doi.org/10.3390/M2010
APA StyleKahnert, S. R., & Schmidt, A. (2025). Synthesis of Mesoionic 1,3,4-Thiadiazole-2-Thiolates. Molbank, 2025(2), M2010. https://doi.org/10.3390/M2010






