Abstract
Because of the continuous interest in ferrocene chemistry, there is a sustained demand for various ferrocenic building blocks, especially small molecules with useful chemical functional groups, sometimes containing multiple groups. Our interest in ferrocene ketoesters (ω-ferrocenyl-ω-ketoesters) was motivated by the synthesis of esters and subsequently alcohols of ferrociphenols. However, from a bibliographic survey, only one publication dated from 1964 reports the two-step synthesis (six-step synthesis from ferrocene) of methyl 2-ferrocenyl-2-oxoacetate, the simplest member of this family of compounds, with no further developments since. We hypothesized that a simpler method might exist, such as the Friedel–Crafts method. By focusing on our experiments to use aluminum trichloride as the catalyst, we managed to achieve the synthesis of FcCOCOOMe in a single step, albeit with a very low yield, regardless of reaction time, temperature, amount of aluminum chloride and reagents concentration. Nevertheless, considering the time saved, simplicity, and the use of less hazardous and less expensive reagents, this method offers certain advantages for synthesizing this building block.
1. Introduction
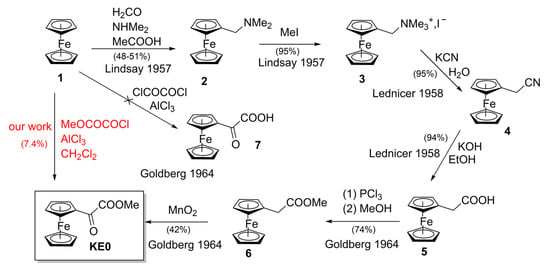
Recent years have seen significant advances in ferrocene chemistry [1,2]. Ferrocene compounds have diverse applications, including anticancer agents [3,4,5], logic gates [6], rocket propellant burn rate catalysts [7], flame retardants [8], and more. To synthesize these molecules, various types of ferrocenic building blocks are required. Among these, some are bifunctional, such as ω-ferrocenyl-ω-ketoesters Fc-CO(CH2)nCOOR (Fc = ferrocene). Focusing on articles (excluding patents), most of the methyl ω-ferrocenyl-ω-ketoesters series KEn (n ranging from 0 (KE0) to 15 (KE15)) have been documented (Table 1). As part of our research on ferrocenic anticancer molecules, we needed several of these esters, particularly KE1, KE2, KE3, and KE4, to synthesize esters of ferrocidiphenols, precursors of highly potent alcohols [9,10]. We needed α-ketoester KE0, first published in 1964 [11], without further references, to prepare the shorter alcohol of this series. In accordance with the six steps beginning with ferrocene 1 (Scheme 1), it is necessary to first prepare amine 2, and subsequently the quaternary ammonium salt 3 [12]. This precursor, which is now conveniently available commercially, was utilized in our preparation of Fc-CH2-imide as well [13]. Salt 3 was utilized to synthesize nitrile 4, which was then converted to acid 5 [14]. This acid was subsequently transformed into its ester 6 and oxidized in KE0 [11], achieving an overall yield of approximately 13%.

Table 1.
Bibliographic survey on methyl ω-ferrocenyl-ω-ketoesters KE0–15 synthesis (0 ≤ n ≤ 15, excluding patents).
Scheme 1.
Synthesis of methyl 2−ferrocenyl−2−oxo−acetate KE0 in six steps and our synthesis in one step using Friedel–Crafts method (in red). References: Goldberg 1964 [11], Lindsay 1957 [12], Lednicer 1958 [14].
It seemed important to us to publish a simpler method to synthesize KE0, as nothing has changed in 61 years [11] regarding this building block. Additionally, NMR was not utilized as it is today, and no spectrum was provided at that time, nor after. In 1964, Goldberg et al. were interested in ω-ferrocenyl-ω-ketoacids, including α-ketoacid 7. They attempted to prepare 7 by direct reaction of oxalyl chloride with ferrocene 1 [11] (Scheme 1), but this approach failed. They did not pursue any further, and instead focused their efforts on ketoester KE0, which is the main subject of their article (along with the precursor ester 6). They also did not describe the saponification of KE0 to 7, possibly due to concerns about decarboxylation or degradation (KE0 is not very stable in solution; see below). Surprisingly, after attempting a Friedel–Crafts reaction with oxalyl chloride (ClCOCOCl) to prepare 7, they did not try using this method to prepare KE0 in a simpler way using methyl chlorooxoacetate (MeOCOCOCl), a closely related reagent already known at the time (referred to as methoxalyl chloride when KE0 was called methoxalylferrocene). We successfully tested this reaction, but with an extremely low yield. Nevertheless, it offers chemists an alternative method to replace six steps with just one when time and simplicity are the primary criteria being sought.
2. Results and Discussion
2.1. Synthesis of KE0 and Study of the Conditions
The standard conditions for the Friedel–Crafts coupling reaction (ferrocene as the aromatic compound, methyl chlorooxoacetate as the acid chloride, aluminum trichloride as the catalyst, and dry dichloromethane as the solvent; Scheme 2) were applied in several runs with variations in the experimental conditions. Regardless of the conditions, significant degradation (black color, very polar matter) was observed along with very low yields for KE0 (<8%). This compound has a strong red color, and is easily identifiable on the TLC plate. Purification was carried out using chromatography on silica gel (prepacked columns) with dichloromethane as the eluent. Dichloromethane stabilized with ethanol should be avoided, as it slightly increases the elution speed and results in poorer separation compared to ethanol-free dichloromethane. However, KE0 was not 100% pure after chromatography, as NMR showed a small amount of impurity with signals around 4.2 ppm (Figure S4). Goldberg et al. noted in their publication [11] that KE0 was unstable during chromatography on alumina, and did not attempt chromatography on silica gel, opting for three consecutive distillations instead. We cannot confirm that KE0 is unstable on silica gel due to the short time it remained in the column (<15 min). Nonetheless, KE0 showed clear signs of degradation in solution over longer periods, even without contact with silica gel. Indeed, when KE0 was left in dichloromethane for a few days at room temperature, a black precipitate appeared, and a gray deposit adhered to the glass. As a test, the 1H NMR spectrum of (impure) KE0 in chloroform was measured at day 0 and day 14 (kept at room temperature), showing a significant loss of resolution, even for the CHCl3 peak (Figure S4). Consequently, attempts to crystallize KE0 at room temperature with slow solvent evaporation to obtain crystals suitable for XRD failed. Ultimately, to obtain pure KE0, subsequent recrystallization was required at lower temperature. This was performed in a freezer (around −30 °C, overnight) using a mixture of diethyl ether and pentane, but without obtaining crystals.
Scheme 2.
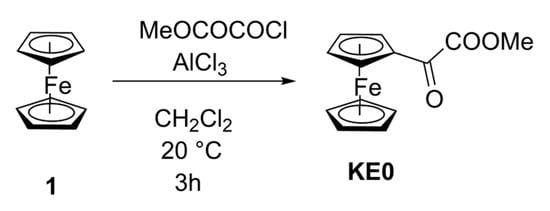
Synthesis of compound KE0 (a more detailed version of the red part of Scheme 1).
Regarding the reaction itself, several experiments were performed starting from 2 g of chlorooxoacetate and 1.5 equiv. of ferrocene, with variation in temperature, time, concentration, and quantity of AlCl3 (Table 2, entries 1–8). Despite our efforts, the best yield achieved was 7%, and a high degradation was always present. To improve these disappointing results, we adapted a procedure used by Turbitt and Watts [26] to prepare [3]ferrocenophan-1-one, a ketone we used to synthesize highly potent [3]ferrocenophanes via McMurry coupling reaction [27]. The conditions for preparing [3]ferrocenophan-1-one were similar to those for KE0 (Friedel–Crafts reaction in dichloromethane, but followed by cyclization here), with a low yield at 0 °C (but much higher than for KE0; entry 1). They found that the best results (yield 40–60%) were obtained at −78 °C for 6 h, demonstrating that Friedel–Crafts coupling reactions of acyl chlorides on ferrocene can be successful at very low temperatures. We set the reaction time to 24 h, which does not allow for the use of dry ice, so an immersion cooler was mandatory. However, our setup (immersion cooler + ethanol bath + flask) could not reach temperatures lower than −68 °C. We conducted the run at this temperature (entry 9), and although degradation was reduced, the yield was still low, despite the slightly higher temperature and the reaction time being four times longer than in Turbitt and Watts’ experiment. A significant amount of ferrocene was present (based on the area of the first peak during chromatography), indicating that methyl chlorooxoacetate has low reactivity with it. A second test at −29 °C (entry 10) was performed to find the ideal temperature but the yield remained low. The best conditions were eventually those of entry 4, as depicted in Scheme 2.

Table 2.
Study of the conditions for the synthesis of KE0 by Friedel–Crafts reaction.
2.2. Characterization of KE0
The structure of ketoester KE0 was determined by several classical methods, including 1H NMR (Figure S1), 13C NMR (Figure S2), DEPT135 (Figure S3), mass spectrometry (Figures S5 and S6), and infrared spectroscopy (Figure S7). For infrared spectroscopy, Golberg et al. [11] used a chloroform solution, whereas we used Attenuated Total Reflectance (ATR) with solid KE0. They reported signals at 5.74 µ (1742 cm−1) for the COO group and 5.99 µ (1669 cm−1) for the ketone group. We found similar values (1728 and 1659 cm−1, respectively). The 1H NMR spectra (Figure S1, no NMR data provided by Golberg et al. [11]) showed a few signals: two doublets (2H and 2H, C5H4) and a singlet (5H, Cp) characteristic of ferrocene, along with a singlet (3H) for the methyl group. The 13C NMR (Figure S2) also displayed signals corresponding to ferrocene (including its quaternary carbon), the methyl group, and the two carbonyl groups (ketone and ester, disappearing in DEPT135; Figure S3). The molecular formula was initially confirmed by low-resolution mass spectrometry (chemical ionization by ammonia, giving [M + H]+ and [M + NH4]+, Figure S5), and subsequently by HRMS (Figure S6).
3. Materials and Methods
3.1. General Procedure
1H and 13C-NMR spectra were acquired using a Bruker 300 spectrometer (Bruker France, Wissembourg, France; s = singlet; d = doublet; t = triplet; q = quadruplet, m = multiplet). CI-MS was performed using a DSQ II spectrometer using chemical ionization with NH3 (Thermo Fisher Scientific, Illkirch, France). High-resolution mass spectrometry (HRMS) was performed at the MS3U platform of Sorbonne Université (Paris, France). IR spectra were recorded on a FT-IR spectrometer (Tensor 27, Bruker France, Wissembourg, France) equipped with an ATR MIRacle accessory (Pike Technologies inc., Madison, WI, USA). Thin-layer chromatography was performed on silica gel 60 GF254 (Merck KGaA, Darmstadt, Germany). Prepacked silica gel columns were obtained from Grace (Grace, Columbia, MD, USA). Flash chromatography was performed using a PuriFlash XS520plus apparatus (Interchim, Montluçon Cedex, France). Experiments at low temperature were performed using a Neslab Cryocool CC-100 immersion cooler (Neslab Instrument inc., Newington, NH, USA). Methyl chlorooxoacetate was obtained from Fluorochem Ltd. (Fluorochem Ltd., Unit 14, Hadfield, UK). Other reagents were obtained from Sigma Aldrich (Saint-Quentin-Fallavier Cedex, France) and used as received.
3.2. Synthesis of Methyl 2-Ferrocenyl-2-oxo-acetate KE0
Ferrocene (CAS 7446-70-0, 4.556 g, 24.5 mmol, 1.5 equiv.) was dissolved in dry dichloromethane (60 mL) and stirred at room temperature. Aluminum trichloride (CAS 7446-70-0, 3.265 g, 24.5 mmol, 1.5 equiv.) was added in portions over 5 min, and the flask was placed under argon. Methyl chlorooxoacetate (CAS 5781-53-3, 2 g, 16.3 mmol) was then added using a syringe over 10 min, and stirring continued for 3 h. The solution was slowly poured into water containing a small amount of hydrochloric acid and decanted. The aqueous layer was extracted with dichloromethane, and the combined organic layers were washed with water, dried over magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in a small quantity of dichloromethane, followed by filtration through a filter containing 3–4 cm of silica gel, eluting with dichloromethane until the filtrate lost its red color. The filtrate was then concentrated under reduced pressure and subjected to chromatography on silica gel (using a prepacked column and automatic flash chromatography apparatus) with ethanol-free dichloromethane as the eluent, yielding methyl 2-ferrocenyl-2-oxo-acetate (KE0) as a red solid (0.328 g), with a yield of 7.4%.
1H NMR (300 MHz, CDCl3): δ 1.95 (s, 3H, CH3), 4.27 (s, 5H, Cp), 4.71 (t, J = 2.0 Hz, 2H, C5H4), 5.03 (t, J = 2.0 Hz, 2H, C5H4). 13C NMR (75 MHz, CDCl3): δ 52.8 (CH3), 70.7 (5CH, Cp), 71.2 (2CH, C5H4), 74.51 (2CH, C5H4), 74.54 (C, C5H4), 163.6 (COO), 188.3 (CO). IR (ATR, ν cm−1): 1728 (COO), 1659 (CO). MS (CI, NH3) m/z: 273 [M + H]+, 290 [M + NH4]+. HRMS (ESI, C13H12FeNaO3: [M + Na]+) calcd: 295.0028, found: 295.0024.
4. Conclusions
Using the Friedel–Crafts reaction, we have simplified the preparation of KE0 by replacing the six-step synthesis published 61 years ago with a single reaction, reducing the number of reagents, cost, and chemical waste. For instance, the formation of nitrile 4 involved the use of highly toxic potassium cyanide, while the oxidation of 6 to KE0 used manganese dioxide, which is also a hazardous reagent. Although the yield (7.4%) is very low and does not surpass the overall yield of the six-step synthesis, our single-step synthesis uses inexpensive reagents like ferrocene, aluminum chloride, and methyl chlorooxoacetate in dichloromethane, without heating or cooling, and is incomparable in terms of time efficiency. The synthesis and purification can be completed in a single day. Some intermediates are now commercially available, but this increases the cost. Additionally, a portion of ferrocene could possibly be recovered and recycled (≈1.8 g for entry 4). The low yield appears to be due to the low reactivity of methyl chlorooxoacetate towards ferrocene, as well as side reactions leading to significant degradation. At very low temperature, the low yield seems essentially due to absence of reaction, while at positive temperature, degradation is probably predominant. Based on our experience on Friedel–Crafts coupling reactions, the behavior of methyl chlorooxoacetate differs from other acyl chlorides with n > 0 (having methylene groups between the two carbonyl groups), which readily undergo Friedel–Crafts coupling with ferrocene. We also prepared the ethyl version of KE0 using the same method, and obtained a similar yield (potentially publishable as a precursor later). This study was limited to using AlCl3, but other Lewis acids could be tested, such as anhydrous zinc chloride, anhydrous iron chloride, boron trifluoride, or even metal oxides like ZnO or other reagents, provided they do not favor the degradation of KE0.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1. 1H NMR spectrum (300 MHz) of compound KE0 in CDCl3; Figure S2. 13C NMR spectrum (75 MHz) of compound KE0 in CDCl3; Figure S3. Stacking of 13C NMR + DEPT135 spectra (75 MHz, CDCl3) of compound KE0; Figure S4. Stacking of 1H NMR spectra (300 MHz, CDCl3) of compound KE0 on day 0 and day 14; Figure S5. Low-resolution mass spectrum of compound KE0 (chem. ionization, NH3); Figure S6. Zoomed-in view of the HRMS spectrum of KE0 (m/z: range 290–300); Figure S7. Infrared spectrum of compound KE0 (ATR).
Author Contributions
Conceptualization, P.P.; methodology, P.P. and H.H.; validation, P.P. and H.H.; formal analysis, P.P.; investigation, P.P. and H.H.; data curation, P.P.; writing—original draft preparation, P.P.; writing—review and editing, P.P. and H.H.; visualization, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agence Nationale de la Recherche (ANR) under grant number ANR-06-BLAN-0384–01, “FerVect”.
Data Availability Statement
The data are contained within this article and the Supplementary Materials.
Acknowledgments
The author thanks Michèle Salmain for checking this manuscript, Céline Fosse for the low-resolution mass spectra.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rauf, U.; Shabir, G.; Bukhari, S.; Albericio, F.; Saeed, A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules 2023, 28, 5765. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Astruc, D. Electron-reservoir applications of ferrocenes and other late transition-metal sandwich complexes: Flow batteries, sensing, catalysis, and biomedicine. Coord. Chem. Rev. 2025, 524, 216300. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti-cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The Medicinal Chemistry of Ferrocene and Its Derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Tomar, V.; Kumar, P.; Sharma, D.; Joshi, R.K.; Nemiwal, M. Anticancer potential of ferrocene-containing derivatives: Current and future prospective. J. Mol. Struct. 2025, 1319, 139589. [Google Scholar] [CrossRef]
- Tzeliou, C.E.; Zois, K.P.; Tzeli, D. Molecular Logic Gates Based on Ferrocene-Containing Compounds. Inorganics 2024, 12, 106. [Google Scholar] [CrossRef]
- Valdebenito, C.; Gaete, J.; Osorio, C.; Dibdalli, Y.; Norambuena, A.; Lecaros, N.; Carrasco, C.; Reyes, H.; Abarca, G.; Morales-Verdejo, C. Evaluation of Mono and Bimetallic Ferrocene-Based 1,2,3-Triazolyl Compounds as Burning Rate Catalysts for Solid Rocket Motor. ACS Omega 2023, 8, 35242–35255. [Google Scholar] [CrossRef] [PubMed]
- Carty, P.; Grant, J.; Simpson, A. Synthesis of a novel ferrocene derivative having flame-retardant and smoke-suppressant properties. Appl. Organomet. Chem. 1988, 2, 277–280. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Xie, P.-P.; Yang, S.; Pigeon, P.; Xiong, Y.; Gai, S.; Qi, X.; Wang, J.; Zhang, Q.; et al. Deciphering the Diversified Metabolic Behavior of Hydroxyalkyl Ferrocidiphenols as Anticancer Complexes. J. Med. Chem. 2023, 67, 1209–1224. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. Int. Ed. 2015, 54, 10230–10233. [Google Scholar] [CrossRef]
- Goldberg, S.I.; Keith, L.H. Synthesis of Methoxalylferrocene. J. Chem. Eng. Data 1964, 9, 250–251. [Google Scholar] [CrossRef]
- Lindsay, J.K.; Hauser, C.R. Aminomethylation of ferrocene to form N,N-dimethylaminomethylferrocene and its conversion to the corresponding alcohol and aldehyde. J. Org. Chem. 1957, 22, 355–358. [Google Scholar] [CrossRef]
- Pigeon, P.; Gaschard, M.; Othman, M.; Salmain, M.; Jaouen, G. α-Hydroxylactams as Efficient Entries to Diversely Functionalized Ferrociphenols: Synthesis and Antiproliferative Activity Studies. Molecules 2022, 27, 4549. [Google Scholar] [CrossRef]
- Lednicer, D.; Lindsay, J.K.; Hauser, C.R. Reaction of the methiodide of N,N-dimethylaminomethylferrocene with potassium cyanide to form ferrocylacetonitrile. J. Org. Chem. 1958, 23, 653–655. [Google Scholar] [CrossRef]
- Ge, F.; Ye, H.; Luo, J.-Z.; Wang, S.; Sun, Y.-J.; Zhao, B.-X.; Miao, J.-Y. A new fluorescent and colorimetric chemosensor for Cu(II) based on rhodamine hydrazone and ferrocene unit. Sens. Actuators B Chem. 2013, 181, 215–220. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Gu, Z. BF3·OEt2-Promoted Synthesis of 2,3-Metallocenocyclohexanones: A 1,2-Hydride Shift and Cationic Cyclization Strategy. J. Org. Chem. 2015, 80, 7865–7875. [Google Scholar] [CrossRef]
- Zherebker, K.Y.; Rodionov, A.N.; Pilipenko, E.S.; Kachala, V.V.; Nikitin, O.M.; Belousov, Y.A.; Simenel, A.A. The synthesis of ferrocenyl- and ferrocenoylpyrimidines. Russ. J. Org. Chem. 2014, 50, 1150–1154. [Google Scholar] [CrossRef]
- Lee, B.C.; Choe, Y.S.; Chi, D.Y.; Paik, J.-Y.; Lee, K.-H.; Choi, Y.; Kim, B.-T. 8-Cyclopentadienyltricarbonyl 99mTc 8-Oxooctanoic Acid: A Novel Radiotracer for Evaluation of Medium Chain Fatty Acid Metabolism in the Liver. Bioconjugate Chem. 2004, 15, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Tachi, M. Effect of the ferrocene nucleus on the microwave-accelerated esterification reaction. Int. J. Chem. 2023, 15, 26–33. [Google Scholar] [CrossRef]
- Wieczorek, A.; Blauz, A.; Makal, A.; Rychlik, B.; Plazuk, D. Synthesis and evaluation of biological properties of ferrocenyl-podophyllotoxin conjugates. Dalton Trans. 2017, 46, 10847–10858. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H. Synthesis and biological evaluation of fatty acids conjugates bearing cyclopentadienyl-donors incorporated [99mTc/Re(CO)3]+ for myocardial imaging. Eur. J. Med. Chem. 2014, 72, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wilbert, G.; Traud, S.; Zentel, R. Liquid crystalline main-chain polymers containing the ferrocene unit as a side group, 2. Variation of the spacer length. Macromol. Chem. Phys. 1997, 198, 3769–3785. [Google Scholar] [CrossRef]
- Uehara, T.; Uemura, T.; Hirabayashi, S.; Adachi, S.; Odaka, K.; Akizawa, H.; Magata, Y.; Irie, T.; Arano, Y. Technetium-99m-Labeled Long Chain Fatty Acid Analogues Metabolized by β-Oxidation in the Heart. J. Med. Chem. 2007, 50, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Seiders, R.P.; Brookhart, M.; Whitten, D.G. Synthesis and reactivity of a surfactant ferrocene. Environmental effects on oxidation of ferrocene in organized media. Isr. J. Chem. 1980, 18, 272–278. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, D.H.; Lee, I.; Choe, Y.S.; Chi, D.Y.; Lee, K.-H.; Choi, Y.; Kim, B.-T. 16-Cyclopentadienyl Tricarbonyl 99mTc 16-Oxo-hexadecanoic Acid: Synthesis and Evaluation of Fatty Acid Metabolism in Mouse Myocardium. J. Med. Chem. 2008, 51, 3630–3634. [Google Scholar] [CrossRef]
- Turbitt, T.D.; Watts, W.E. Bridged ferrocenes: XII. The synthesis of [3] ferrocenophan-1-one from ferrocene by a novel one-step annelation reaction. Organomet. Chem. 1972, 46, 109–117. [Google Scholar] [CrossRef]
- Görmen, M.; Pigeon, P.; Top, S.; Hillard, E.A.; Huché, M.; Hartinger, C.G.; de Montigny, F.; Plamont, M.-A.; Vessières, A.; Jaouen, G. Synthesis, Cytotoxicity, and COMPARE Analysis of Ferrocene and [3]Ferrocenophane Tetrasubstituted Olefin Derivatives against Human Cancer Cells. ChemMedChem 2010, 5, 2039–2050. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).