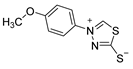
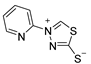
Abstract
A reliable synthesis of C5-unsubstituted 1,3,4-thiadiazole-2-thiolates is described that avoids potentially explosive or laborious steps. This work presents a reliable method for preparing the starting material dithioformate from carbon disulfide and potassium or sodium tri-sec-butylhydroborates for the preparation of the mesoionic title compounds with potassium hydrazinecarbodithioates. New 1,3,4-thiadiazole-2-thiolates are presented, and missing structural analysis data of known derivatives are added (1D- and 2D-NMR, HR-ESI-MS, IR).
1. Introduction
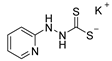
The first 1,3,4-thiadiazole-2-thiolate 1 was mentioned as early as 1895 by Busch et al. [1], making this compound one of the oldest representatives of mesoionic compounds [2]. These form a subgroup of the class of mesomeric betaines [3], which are characterized by an equal number of positive and negative charges delocalized within a common π-electron system of a heterocyclic ring system without any non-charged mesomeric structure. The oldest mesoionic compound mentioned in the literature is dehydrodithizone 2 (see Figure 1), which was described by Fischer and Besthorn as early as 1882 [4,5]. The most frequently cited representatives of mesoionic compounds in the literature, however, are derivatives from the sydnone and münchnone family, such as sydnones 3 [6,7,8], sydnon-imines 4 [8,9], sydnone-methides 5 [10], münchnones 6 [11], or thioisomünchones 7 [12]. This is due to their synthetic potential as masked 1,3-dipoles [11,12], N-heterocyclic carbene precursors [9,10], or their biological activities [6,7].
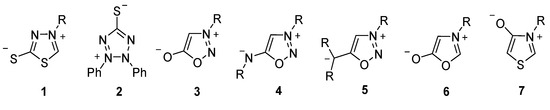
Figure 1.
Examples of mesoionic compounds.
Among these examples, C5-unsubstituted 1,3,4-thiadiazole-2-thiolates in particular seem to be the least studied, perhaps because of their difficult synthetic accessibility to date. Nevertheless, this ring system was met with great interest. 1,3,4-Thiadiazole-2-thiolates were calculated in terms of their aromaticity [13] as well as their suitability as precursors for the formation of N-heterocyclic carbenes [14], and patented for several applications, e.g., in lithography [15]. Electronic absorption spectra [16], mass spectra [17], and electric dipole moments [18] were examined. So far, syntheses have almost exclusively led to 1,3,4-thiadiazole-2-thiolates that are substituted in the 5-position. For example, reactions of hydrazine-1-carbodithioates with acyl chlorides or aldehydes [19,20,21], syntheses starting from thiohydrazides and CS2, or, less effectively, thiophosgene, and from hydrazides and carbon disulfide were described [22,23]. The often light- and moisture-sensitive dithioformates M+ HCS2− are needed to synthesize C5-unsubstituted derivatives of 1,3,4-thiadiazol-2-thiolates. Dithioformates are available, for example, from chloroform in a methanolic solution of potassium sulfide under the exclusion of air [24]. According to the literature references, the dithioformates crystallize after concentrating the solution [25,26]. However, this reaction is warned against. It can cause explosions [27] and has been found to be unpredictable, as two out of three attempts lead to an eruption or explosion of the flask contents [28]. A modified version of the reaction was developed, but it requires very careful control of the reaction conditions to be safe [28]. Alternatively, a synthesis is described in which methanol is first reacted with elemental potassium and then treated with hydrogen sulfide, the excess of which must be removed with N2 under reduced pressure. After the addition of more methanol and potassium, chloroform is added dropwise, which, after extensive work-up, yields potassium dithioformate [29]. Another synthesis reduces CS2 with potassium tri-isopropylborohydride in THF [30]. Finally, it is mentioned in the literature that sodium dithioformate is obtained as a by-product when CS2 reacts with NaBH4 (or NaH) with carbon disulfide in the presence of tertiary amines during the preparation of amine-borane adducts [31]. The dithioformate, however, seemingly was not isolated. We report here a simple preparation of potassium and sodium dithioformate and use it in the synthesis of C5-unsubstituted 1,3,4-thiadiazole-2-thiolates. This method is effective and avoids handling elemental potassium or hydrogen sulfide.
2. Results and Discussion
The mesoionic 1,3,4-thiadiazole-2-thiolates, which were prepared in this work, were accessible via the reaction route shown in Scheme 1. Retrosynthetically, the heterocycles can be reduced to the corresponding N-arylated hydrazines 9a–d, carbon disulfide, and the C1-building block dithioformate 11a,b mentioned above (see Scheme 1).
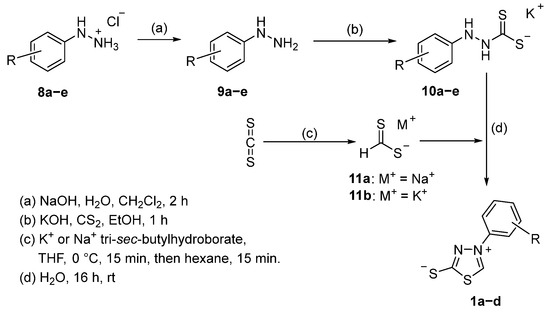
Scheme 1.
Synthetic pathway of the title compounds.
The required hydrazines were obtained as hydrochlorides 8a–d directly from the supplier and reacted with NaOH in aqueous solution to the corresponding free hydrazines 9a–e [32] and, after extraction with DCM and drying over MgSO4, were used for the subsequent reactions without further purification. Said hydrazines were dissolved in ethanolic potassium hydroxide solution and reacted with carbon disulfide [33]. The deprotonation of hydrazines or amines in the presence of a strong base with subsequent addition of CS2 is a synthetic approach for the formation of thioamides that is also used in modern literature [34]. The separate isolation of the free hydrazines was chosen since the direct treatment of the hydrochlorides with an excess of KOH and subsequent addition of CS2 would result in inorganic side-products, such as KCl, which are unnecessarily hard to remove from the water-soluble potassium hydrazinecarbodithioates. During the addition of the disulfide, the corresponding potassium salts 10a–e started to precipitate and the addition of diethyl ether (Et2O) completed the precipitation. The resulting solids were filtered off and thoroughly washed with dichloromethane to remove any residual hydrazine, which usually results in analytically pure products. If necessary, the potassium salts 10a–e can be recrystallized in methanolic solution and were isolated in good to very good yields (see Table 1).

Table 1.
Isolated potassium salts 10a–e.
The second building block of the synthesis route is synthesized via the reduction of CS2 with tri-sec-butylhydroborates (Selectrides, Merck) in short reaction times and moderate yields. A 1 M solution of potassium or sodium tri-sec-butylhydroborates in abs. THF was added dropwise to a solution of CS2 in abs. THF under ice cooling, which led to the formation of an intensely yellow solid. After dilution of the suspension with hexane, filtration, and extensive washing with hexane, the dithioformates 11a,b were obtained as analytically pure solids. Described syntheses of the dithioformates by reduction of carbon disulfide with either KB(OiPr)3H, NaBH4, or NaH (vide supra) were not successful in our case. The mesoionic compounds 1a–e were then smoothly obtained via the reaction of the corresponding potassium salts 10a–e with the dithioformates 11a,b in an aqueous medium. No significant difference in yield and reaction time was observed for the usage of either potassium or sodium dithioformate, and syntheses of the mesoionic compounds were conducted with the potassium dithioformate 11b for the sake of uniform cations in the reaction. The mesoionic compounds precipitated from the aqueous solution, with the exception of 1e (vide infra), and can thus be separated from their water-soluble starting materials after filtration and thorough washing with water. Hence, the described mesoionic heterocycles 1a–d can be isolated in analytical purity without complex work-up. For rapid removal of residual water, the heterocycles were redissolved in MeOH, and the solvent was removed under reduced pressure. This process was repeated three times, and the samples were dried to constant weight in a fine vacuum before gravimetric determination of the yield (see Table 2).

Table 2.
Isolated mesoionic compounds 1a–e.
In contrast to the fast conversion of potassium salts 10a–d for the syntheses of the heterocycles 1a–d, no conversion to the anticipated mesoion 1e was observed. Instead, a ring-closure reaction yielding [1,2,4]triazolo[4,3-a]pyridine 12 was observed following a competitive nucleophilic attack of the nitrogen of the pyridine ring at the dithioformate moiety, as described in the proposed mechanism in Scheme 2 (mechanism A). This stands in analogy to literature procedures for the syntheses of 12 via, e.g., base-promoted oxidative iodination of the corresponding hydrazone [35] or the addition of C1-building blocks such as formic acid to the corresponding hydrazine [36]. However, to the best of our knowledge, this is the first reported case of the isolation of 12 starting from thioformates. In addition to the formation of the bicyclic system 12, conversion of the potassium salt 10e to [1,2,4]triazolo[4,3-a]pyridine-3-thiol 13 was achieved by stirring 10e in an aqueous solution at 100 °C for 48 h without the addition of dithioformates 11a,b (Scheme 2, mechanism B), in analogy to the direct addition of carbon disulfide to 2-hydrazinepyridine 9e in aqueous solution as described in the literature [37].
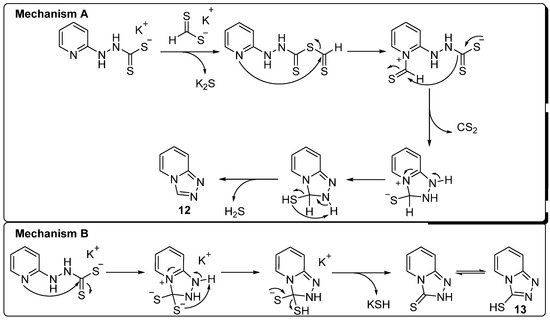
Scheme 2.
Proposed mechanisms for the formation of 12 and 13.
3. Materials and Methods
3.1. General Experimental Information
Commercially available materials were used without further purification unless noted otherwise. Solvents were dried according to established procedures and stored under nitrogen. The reaction progress was monitored by thin-layer chromatography (TLC) on silica-coated aluminum sheets from Merck (Darmstadt, Germany; 60 F254). NMR spectra were measured on the FT-NMR Devices Avance Neo (400 MHz) and Avance III (600 MHz) from Bruker (Ettlingen, Germany) and analyzed with TopSpin 3.5 from Bruker. Chemical shifts (δ) are given in ppm relative to the residual solvent d6-DMSO (1H (d6-DMSO): δ = 2.50 ppm, 13C (d6-DMSO): δ = 39.52 ppm) [38]. Coupling constants J are given in hertz (Hz), with the following abbreviations for multiplicity: s (singlet), d (doublet), dd (doublet of doublet), ddd (doublet of doublet of doublet), dt (doublet of triplet), t (triplet), and td (triplet of doublet). Signals without analyzable coupling are marked as m (multiplet). The prefix app. (apparent) is used to highlight that the observed multiplicity is a result of vague subordinated coupling. For the 13C/DEPT-spectra, (+) is added for carbons bonded to one or three hydrogens and (−) for carbons bonded to two hydrogens. Quaternary carbon atoms are denoted with the symbol (o). The hydrazines were directly purchased from the supplier (Merck, TCI) as free base or as the hydrochloride and, if necessary, converted to the free hydrazine according to literature procedures ([32], general procedure 1, vide infra) and were used without further purification.
3.2. Experimental Procedures and Characterization of Compounds
- (1)
- General procedure for the conversion of hydrazine hydrochlorides to free hydrazines
To a solution of the corresponding hydrochloride (1 equiv.) in H2O (10 mL/mmol), NaOH (2 equiv./equiv. of HCl) and DCM (8 mL/mmol) were added, and the solution was vigorously stirred at rt for 2 h. The organic layer was separated, and the aqueous solution was extracted with DCM (3×). The combined organic layers were washed once with water, dried over MgSO4, and the solvent was removed under reduced pressure after filtration. The obtained free hydrazines were generally not suitable for long-term storage and were used directly without further purification.
- (2)
- General procedure for the synthesis of potassium hydrazinecarbodithioates:
The corresponding free hydrazine (1 equiv.) was added to a solution of KOH (1 equiv.) in abs. EtOH, followed by the dropwise addition of carbon disulfide (2 equiv.) over the course of 15 min at rt (the product may already precipitate during the addition). After complete addition, the solution was stirred for 1 h and diluted with Et2O. The precipitate was collected by filtration, washed with DCM, and recrystallized if necessary. All salts isolated in this work are highly hygroscopic.
- (3)
- General procedure for the synthesis of 1,3,4-thiadiazolium-2-thiolates:
A solution of the corresponding potassium hydrazinecarbodithioate (1 equiv.) in water (5 mL/mmol) was treated with potassium dithioformate and stirred at rt for 16 h. The precipitated mesoionic compound was collected by filtration, thoroughly washed with water, and dried under vacuum. For optimized yields, the use of freshly prepared potassium hydrazinecarbodithioates and dithioformates is advised.
3.2.1. Sodium Dithioformate (Sodium Methanedithioate) (11a)
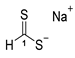
Sodium tri-sec-butylhydroborate (1 M solution in abs. THF, 10.000 g, 11.20 mL, 48.51 mmol) was added dropwise over the course of 15 min. at 0 °C with a N2-purged syringe to a stirred solution of carbon disulfide (7.386 g, 5.83 mL, 97.01 mmol) in abs. THF (10 mL). After complete addition, n-hexane (50 mL) was added and stirring was continued for an additional 15 min. The precipitated salt was isolated by filtration, thoroughly washed with n-hexane, and dried under vacuum to obtain 11a (2.219 g, 22.16 mmol, 45%) as a yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 12.03 (s, 1H, H-1) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 245.8 (+, 1C, C-1) ppm. M.P.: 74–76 °C; IR (ATR) v = 3181, 2886, 2103, 1634, 1428, 1189, 1139, 1123, 1049, 998, 884, 767, 739, 682, 498, 473 cm−1; HR-ESI-MS: calcd. for [C2H2S4Na]− (dimeric sodium salt): 176.8943; found: 176.8938.
3.2.2. Potassium Dithioformate (Potassium Methanedithioate) (11b)
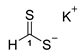
Potassium tri-sec-butylhydroborate (1 M solution in abs. THF, 10.000 g, 10.95 mL, 45.00 mmol) was added dropwise over the course of 15 min at 0 °C with a N2-purged syringe to a stirred solution of carbon disulfide (6.851 g, 5.41 mL, 89.99 mmol) in abs. THF (10 mL). After complete addition, n-hexane (50 mL) was added, and stirring was continued for an additional 15 min. The precipitated salt was isolated by filtration, thoroughly washed with n-hexane, and dried under vacuum to obtain 11b (2.162 g, 18.60 mmol, 41%) as a yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 12.04 (s, 1H, H-1) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 245.8 (+, 1C, C-1) ppm. M.P.: 69–71 °C; IR (ATR) v = 3277, 2102, 1626, 1590, 1377, 1349, 1262, 1193, 1114, 1039, 993, 884, 833, 768, 733, 667, 581, 529 cm−1; HR-ESI-MS: calcd. for [C2H2S4K]− (dimeric potassium salt): 192.8682; found: 192.8676.
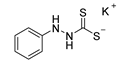
3.2.3. Potassium Phenylhydrazinecarbodithioate (10a)
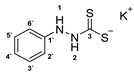
According to general procedure (2), phenylhydrazine (1.000 g, 0.91 mL, 9.25 mmol) was added to a solution of KOH (0.512 g, 9.25 mmol) in abs. EtOH (25 mL), followed by the addition of carbon disulfide (1.408 g, 1.12 mL, 18.50 mmol). Full precipitation was achieved by the addition of Et2O (50 mL). Compound 10a (1.882 g, 8.46 mmol, 91%) was isolated as a colorless solid. If necessary, recrystallization of 10a was achieved from MeOH.
1H-NMR (600 MHz, d6-DMSO): δ = 10.01 (d, J = 5.2 Hz, 1H, H-2), 7.80 (d, J = 5.2 Hz, 1H, H-1), 7.12 (app. ddd, J = 11.6; 6.1; 4.2 Hz, 2H, H-3`, H-5`), 6.75 (app. dd, J = 8.5; 1.0 Hz, 2H, H-2`, H-6`), 6.71–6.76 (m, 1H, H-4`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 212.6 (o, 1C, C-3), 149.6 (o, 1C, C-1`), 128.4 (+, 2C, C-3`, C-5`), 119.1 (+, 1C, C-4`), 114.1 (+, 2C, C-2`, C-6`) ppm. M.P.: 136–138 °C (decomposition); IR (ATR) v = 3372, 3031, 2062, 1596, 1492, 1452, 1407, 1329, 1303, 1232, 1205, 1176, 1122, 1069, 1027, 992, 893, 833, 754, 692, 668, 647, 580, 534, 493 cm−1; HR-ESI-MS: calcd. for [C7H7N2S2]−: 183.0056; found: 183.0054.
The analytical data obtained complement the literature [39].
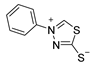
3.2.4. 4-Phenyl-1,3,4-Thiadiazolium-2-Thiolate (1a)
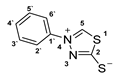
According to general procedure (3), potassium dithioformate (11b, 0.262 g, 2.25 mmol) was added to a solution of potassium phenylhydrazinecarbodithioate (10a, 0.250 g, 1.13 mmol) in water (6 mL). Compound 1a (0.152 g, 0.78 mmol, 69%) was isolated as a light-yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 10.39 (s, 1JC,H = 215.3 Hz, 1H, H-5), 7.92–7.91 (m, 2H, H-3`, H-5`), 7.64–7.63 (m, 3H, H-2`, H-4`, H-6`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 185.1 (o, 1C, C-2), 153.5 (+, 1C, C-5), 138.1 (o, 1C, C-1`), 130.8 (+, 1C, C-4`), 129.8 (+, 2C, C-2`, C-6`), 122.1 (+, 2C, C-3`, C-5`) ppm. M.P.: 193–195 °C; IR (ATR) v = 2979, 2903, 1589, 1491, 1449, 1336, 1310, 1261, 1153, 1100, 1046, 1003, 914, 847, 812, 752, 685, 659, 627, 551, 523, 466 cm−1; HR-ESI-MS: calcd. for [C8H6N2S2+Na]+: 216.9865; found: 216.9864.
The analytical data obtained complement the literature [1].
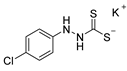
3.2.5. Potassium 1-(4′-Chlorophenyl)Hydrazinecarbodithioate (10b)
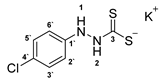
According to general procedure (2), (4-chlorophenyl)hydrazine (1.000 g, 7.01 mmol) was added to a solution of KOH (0.394 g, 7.01 mmol) in abs. EtOH (25 mL), followed by the addition of carbon disulfide (1.068 g, 0.85 mL, 14.03 mmol). Full precipitation was achieved by the addition of Et2O (50 mL). Compound 10b (1.672 g, 6.51 mmol, 92%) was isolated as a colorless solid. If necessary, recrystallization of 10b was achieved from MeOH.
1H-NMR (600 MHz, d6-DMSO): δ = 9.96 (app. s, 1H, H-2), 7.77 (app. s(br), 1H, H-1), 7.15 (d, J = 8.7 Hz, 2H, H-3`, H-5`), 6.73 (d, J = 8.7 Hz, 2H, H-2`, H-3`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 213.7 (o, 1C, C-3), 148.7 (o, 1C, C-1`), 128.1 (+, 2C, C-3`, C-5`), 122.1 (+, 1C, C-4`), 115.3 (+, 2C, C-2`, C-6`) ppm. M.P.: 122–124 °C (decomposition); IR (ATR) v = 3355, 3236, 3176, 2062, 1634, 1594, 1486, 1444, 1313, 1273, 1231, 1207, 1184, 1131, 1087, 1048, 992, 821, 733, 664, 606, 540, 501, 485 cm−1; HR-ESI-MS: calcd. for [C7H6ClN2S2]−: 216.9666; found: 216.9663.
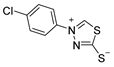
3.2.6. 4-(4′-Chlorophenyl)-1,3,4-Thiadiazolium-2-Thiolate (1b)
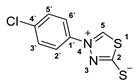
According to general procedure (3), potassium dithioformate (11b, 0.226 g, 1.95 mmol) was added to a solution of potassium 1-(4′-chlorophenyl)hydrazinecarbodithioate (10b, 0.250 g, 0.97 mmol) in water (5 mL). Compound 1b (0.135 g, 0.59 mmol, 60%) was isolated as a light-yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 10.41 (s, 1JC,H = 214.9 Hz, 1H, H-5), 7.95 (d, J = 8.6 Hz, 2H, H-2`, H-6`), 7.72 (d, J = 8.6 Hz, 2H, H-3`, H-5`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 185.1 (o, 1C, C-2), 154.1 (+, 1C, C-5), 136.8 (o, 1C, C-1`), 135.2 (o, 1C, C-4`), 129.8 (+, 2C, C-3`, C-5`), 124.0 (+, 2C, C-2`, C-6`) ppm. M.P.: 201–203 °C; IR (ATR) v = 3436, 3052, 3000, 2938, 2902, 1589, 1493, 1409, 1331, 1230, 1091, 1044, 1009, 851, 820, 720, 640, 608, 580, 52, 496, 471, 416 cm−1; HR-ESI-MS: calcd. for [C8H5ClN2S2+Na]+: 250.9475; found: 250.9474.
The analytical data obtained complement the literature [40].
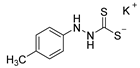
3.2.7. Potassium 1-(P-Tolyl)Hydrazinecarbodithioate (10c)
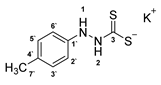
According to general procedure (2), p-tolylhydrazine (1.000 g, 8.19 mmol) was added to a solution of KOH (0.459 g, 8.19 mmol) in abs. EtOH (25 mL), followed by the addition of carbon disulfide (1.246 g, 0.99 mL, 16.37 mmol). Full precipitation was achieved by the addition of Et2O (50 mL). Compound 10c (1.631 g, 6.90 mmol, 84%) was isolated as a colorless solid. If necessary, recrystallization of 10c was achieved from MeOH.
1H-NMR (600 MHz, d6-DMSO): δ = 9.97 (d, J = 3.6 Hz, 1H, H-2), 7.71 (d, J = 3.9 Hz, 1H, H-1), 6.93 (d, J = 7.9 Hz, 2H, H-3`, H-5`), 6.66 (d, J = 8.0 Hz, 2H, H-2`, H-6`), 2.18 (s, 3H, H-7`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 212.1 (o, 1C, C-3), 147.2 (o, 1C, C-1`), 128.8 (+, 2C, C-3`, C-5`), 127.7 (o, 1C, C-4`), 114.4 (+, 2C, C-2`, C-6`), 20.2 (+, 1C, C-7`) ppm. M.P.: 179–181 °C (decomposition); IR (ATR) v = 3367, 3030, 2918, 2861, 2062, 1609, 1508, 1446, 1395, 1380, 1328, 1301, 1245, 1210, 1151, 1111, 1031, 993, 814, 775, 665, 501 cm−1; HR-ESI-MS: calcd. for [C8H9N2S2]−: 197.0213; found: 197.0206.
The analytical data obtained complement the literature [41].
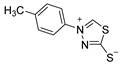
3.2.8. 4-(P-Tolyl)-1,3,4-Thiadiazolium-2-Thiolate (1c)
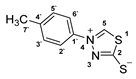
According to general procedure (3), potassium dithioformate (11b, 0.212 g, 2.12 mmol) was added to a solution of potassium 1-(p-tolyl)hydrazinecarbodithioate (10c, 0.250 g, 1.06 mmol) in water (5 mL). Compound 1c (0.157 g, 0.75 mmol, 71%) was isolated as a light-yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 10.33 (s, 1JC,H = 214.8 Hz, 1H, H-5), 7.79 (d, J = 8.6 Hz, 2H, H-2`, H-6`), 7.43 (d, J = 8.3 Hz, 2H, H-3`, H-5`), 2.40 (s, 3H, H-7`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 185.1 (o, 1C, C-2), 152.7 (+, 1C, C-5), 140.8 (o, 1C, C-4`), 135.9 (o, 1C, C-1`), 130.2 (+, 2C, C-3`, C-5`), 121.8 (+, 2C, C-2`, C-6`), 20.7 (+, 1C, C-7`) ppm. M.P.: 191–193 °C; IR (ATR) v = 2985, 2925, 1602, 1504, 1476, 1453, 1334, 1311, 1291, 1257, 1231, 1179, 1164, 1098, 1045, 1020, 1008, 939, 857, 802, 782, 638, 614, 528, 475 cm−1; HR-ESI-MS: calcd. for [C9H8N2S2+Na]+: 231.0021; found: 231.0022.
The analytical data obtained complement the literature [40].
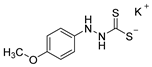
3.2.9. Potassium 1-(4′-Methoxyphenyl)Hydrazinecarbodithioate (10d)
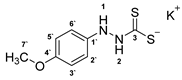
According to general procedure (2), (4-methoxyphenyl)hydrazine (1.000 g, 7.24 mmol) was added to a solution of KOH (0.406 g, 7.24 mmol) in abs. EtOH (25 mL), followed by the addition of carbon disulfide (1.102 g, 0.87 mL, 14.48 mmol). Full precipitation was achieved by the addition of Et2O (50 mL). Compound 10d (1.358 g, 5.38 mmol, 74%) was isolated as a colorless solid. If necessary, recrystallization of 10d was achieved from MeOH.
1H-NMR (600 MHz, d6-DMSO): δ = 10.02 (app. s, 1H, H-2), 7.66 (app. s(br), 1H, H-1), 6.75 (d, J = 9.1 Hz, 2H, H-3`, H-5`), 6.72 (d, J = 9.1 Hz, 2H, H-2`, H-6`), 3.66 (s, 3H, H-7`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 211.7 (o, 1C, C-3), 153.2 (o, 1C, C-4`), 143.3 (o, 1C, C-1`), 115.8 (+, 2C, C-2`, C-6`), 113.9 (+, 2C, C-3`, C-5`), 55.3 (+, 1C, C-7`) ppm. M.P.: 164–166 °C (decomposition); IR (ATR) v = 3376, 3283, 3164, 3000, 2957, 2933, 2834, 2061, 1597, 1543, 1504, 1455, 1439, 1297, 1241, 1170, 1136, 1110, 1021, 874, 825, 725, 625, 577, 511 cm−1; HR-ESI-MS: calcd. for [C8H9N2OS2]−: 213.0162; found: 213.0158.
3.2.10. 4-(4′-Methoxyphenyl)-1,3,4-Thiadiazolium-2-Thiolate (1d)
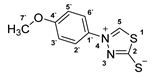
According to general procedure (3), potassium dithioformate (11b, 0.230 g, 1.98 mmol) was added to a solution of potassium 1-(4`-methoxyphenyl)hydrazinecarbodithioate (10d, 0.250 g, 0.99 mmol) in water (5 mL). Compound 1d (0.146 g, 0.65 mmol, 65%) was isolated as a light-yellow solid.
1H-NMR (600 MHz, d6-DMSO): δ = 10.26 (s, 1JC,H = 214.7 Hz, 1H, H-5), 7.85 (d, J = 8.8 Hz, 2H, H-2`, H-6`), 7.15 (d, J = 8.8 Hz, 2H, H-3`, H-5`), 3.84 (s, H, H-7`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 185.0 (o, 1C, C-2), 160.7 (o, 1C, C-4`), 151.8 (+, 1C, C-5), 131.4 (o, 1C, C-1`), 123.6 (+, 2C, C-2`, C-6`), 114.7 (+, 2C, C-3`, C-5`), 55.8 (+, 1C, C-7`) ppm. M.P.: 202–204 °C; IR (ATR) v = 3061, 2968, 2929, 2889, 2837, 2037, 1610, 1593, 1512, 1464, 1450, 1439, 1358, 1346, 1312, 1266, 1182, 1123, 1047, 1025, 1008, 857, 826, 785, 650, 587, 510 cm−1; HR-ESI-MS: calcd. for [C9H8N2OS2+Na]+: 246.9970; found: 246.9970.
3.2.11. Potassium 1-(Pyridine-2-Yl)Hydrazinecarbodithioate (10e)
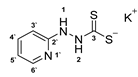
According to general procedure (2), 2-hydrazinopyridine (1.000 g, 9.16 mmol) was added to a solution of KOH (0.514 g, 9.16 mmol) in abs. EtOH (25 mL), followed by the addition of carbon disulfide (1.395 g, 1.11 mL, 18.33 mmol). Full precipitation was achieved by the addition of Et2O (50 mL). Compound 10e (1.361 g, 6.09 mmol, 66%) was isolated as a yellow solid after recrystallization from ethyl acetate.
1H-NMR (600 MHz, d6-DMSO): δ = 9.95 (d, J = 2.6 Hz 1H, H-2), 8.10 (app. s, 1H, H-1), 8.04 (app. dd, J = 4.9; 1.0 Hz, 1H, H-6`), 7.51 (app. td, J = 7.7; 1.8 Hz, 1H, H-4`), 6.69 (app. dt, J = 5.7; 1.3 Hz, 1H, H-3`), 6.61 (app. d, J = 8.4 Hz, 1H, H-5`) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 213.5 (o, 1C, C-3), 160.4 (o, 1C, C-2`), 147.4 (+, 1C, C-6`), 137.2 (+, 1C, C-4`), 114.7 (+, 1C, C-3`), 107.5 (+, 1C, C-5`) ppm. M.P.: 119–121 °C (decomposition); IR (ATR) v = 3537, 3402, 3235, 3139, 2966, 2911, 2898, 1632, 1597, 1573. 1501, 1476, 1443, 1430, 1390, 1325, 1300, 1267, 1147, 1091, 1050, 993, 852, 772, 696, 653. 623, 571, 558, 535, 474 cm−1; HR-ESI-MS: calcd. for [C6H6N4S2]−: 184.0009; found: 184.0007.
3.2.12. [1,2,4]Triazolo[4,3-a]Pyridine (12)
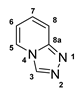
Potassium dithioformate (11b, 0.260 g, 2.24 mmol) was added to a solution of potassium 2-(pyridine-2-yl)hydrazinecarbodithioate (10e, 0.250 g, 1.12 mmol) in water (5 mL) and stirred at 100 °C for 48 h. After cooling to room temperature, the aqueous solution was extracted with DCM (3 × 10 mL) and the combined organic layers were washed with water (1 × 15 mL). After drying the organic solution with MgSO4 and removal of the solvent, 12 (0.042 g, 0.35 mmol, 31%) was isolated as yellow, viscous oil.
1H-NMR (600 MHz, d6-DMSO): δ = 9.26 (s, 1H, H-3), 8.57 (dt, J = 6.9, 1.1 Hz, 1H, H-5), 7.76 (app. dd, J = 9.3, 1.0 Hz, 1H, H-8), 7.35 (ddd, J = 9.3, 6.6, 1.1 Hz, 1H, H-7), 6.96 (td, J = 6.8, 0.8 Hz, 1H, H-6) ppm. 13C-NMR (150 MHz, d6-DMSO): δ = 148.5 (o, 1C, C-8a), 136.5 (+, 1C, C-3), 128.0 (+, 1C, C-7), 125.1 (+, 1C, C-5), 115.0 (+, 1C, C-8), 113.6 (+, 1C, C-6) ppm.
The analytical data obtained are in accordance with the literature [35].
3.2.13. [1,2,4]Triazolo[4,3-a]Pyridine-3-Thiol (13)
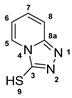
A solution of potassium 2-(pyridine-2-yl)hydrazinecarbodithioate (10e, 0.100 g, 0.45 mmol) in water (5 mL) was stirred at 100 °C for 48 h. After cooling to room temperature, the aqueous solution was extracted with DCM (3 × 10 mL) and the combined organic layers are washed with water (1 × 15 mL). After drying the organic solution with MgSO4 and removal of the solvent, 13 (0.031 g, 0.12 mmol, 46%) was isolated as a yellow solid.
1H-NMR (400 MHz, d6-DMSO): δ = 14.67 (s(br), 1H, H-9), 8.26 (dt, J = 7.1, 1.2 Hz, 1H, H-5), 7.61 (dt, J = 9.4, 1.1 Hz, 1H, H-8), 7.46 (ddd, J = 9.5, 6.5, 1.2 Hz, 1H, H-7), 6.99 (td, J = 7.1, 1.0 Hz, 1H, H-6) ppm. 13C-NMR (100 MHz, d6-DMSO): δ = 159.1 (o, 1C, C-3), 146.2 (o, 1C, C-8a), 131.0 (+, 1C, C-7), 125.0 (+, 1C, C-5), 115.5 (+, 1C, C-8), 113.9 (+, 1C, C-6) ppm.
The analytical data obtained are in accordance with the literature [42].
Supplementary Materials
NMR, IR, and high-resolution MS spectra of all compounds.
Author Contributions
Conceptualization, S.R.K. and A.S.; methodology, S.R.K.; validation, S.R.K. and A.S.; formal analysis, A.S.; investigation, S.R.K.; resources, S.R.K. and A.S.; data curation, S.R.K.; writing—original draft preparation, S.R.K.; writing—review and editing, A.S.; visualization, A.S.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Busch, M. Synthese von Biazolinderivaten. Ber. Dtsch. Chem. Ges. 1895, 28, 2635–2647. [Google Scholar] [CrossRef]
- Ollis, W.D.; Ramsden, C.A. Mesoionic compounds. Adv. Heterocycl. Chem. 2022, 137, 229–347. [Google Scholar]
- Ollis, W.D.; Stanforth, S.P.; Ramsden, C.A. Heterocyclic mesomeric betaines. Tetrahedron 1985, 41, 2239–2329. [Google Scholar] [CrossRef]
- Fischer, E. Ueber die Hydrazinverbindungen. Justus Liebigs Ann. Chem. 1882, 212, 316. [Google Scholar] [CrossRef]
- Kushi, Y.; Fernando, Q. Crystal and molecular structure of the meso-ionic sydnone, anhydro-5-mercapto-2,3-diphenyltetrazolium hydroxide. J. Am. Chem. Soc. 1970, 92, 1965–1968. [Google Scholar] [CrossRef]
- Khan, T.; Yadav, R.; Kesharwani, A.K.; Chourasia, K. A review on synthesis, characterization, and pharmacological properties of some sydnone derivatives. Mini-Rev. Org. Chem. 2025, 22, 359–380. [Google Scholar] [CrossRef]
- Zerbib, S.; Khouili, M.; Catto, M.; Bouissane, L. Sydnone: Synthesis, reactivity and biological activities. Curr. Med. Chem. 2023, 30, 1122–1144. [Google Scholar] [CrossRef]
- Cherepanov, I.; Moiseev, S.K. Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar]
- Freese, T.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sulfur, mercury, and boron adducts of sydnone imine derived anionic N-heterocyclic carbenes. RSC Adv. 2019, 9, 4781–4788. [Google Scholar] [CrossRef]
- Mummel, S.; Lederle, F.; Hübner, E.; Namyslo, J.C.; Nieger, M.; Schmidt, A. Sydnone methides—A forgotten class of mesoionic compounds for the generation of anionic N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2021, 60, 18882–18887. [Google Scholar] [CrossRef]
- Reissig, H.-U. Münchnones—New factes after 50 years. Angew. Chem. Int. Ed. 2014, 53, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Beall, L.S.; Heidelbaugh, T.M.; Liu, B.; Sheehan, S.M. A One-pot bicycloannulation method for the synthesis of tetrahydroisoquinoline systems. J. Org. Chem. 2000, 65, 2684–2695. [Google Scholar] [CrossRef]
- Nein, Y.I.; Morzherin, Y.Y. Criteria for aromaticity of mesoionic heterocycles. Russ. Chem. Bull. 2012, 61, 1111–1116. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Oziminski, W.P. A quantitative analysis of factors influencing ease of formation and σ-bonding strength of oxa- and thia-n-heterocyclic carbenes. J. Org. Chem. 2017, 82, 12485–12491. [Google Scholar] [CrossRef]
- Mitsubishi Paper Mills, Ltd. Japan. Developers Containing 3-Thioxo-1,2-diazole Inner Salts for Lithographic Plate Making by Silver Complex Diffusion-Transfer Process. JP04328559 A, 17 November 1992. [Google Scholar]
- Irving, H.M.N.H.; Kiwan, A.M. Effect of solvent on the electronic absorption spectra of the mesoionic compounds 2,3-diphenyl-2H-tetrazolium-5-thiolate and 4-phenyl-1,3,4-thiadiazolium-2-thiolate. J. Chem. Soc. B 1971, 5, 898–901. [Google Scholar]
- Ollis, W.D.; Ramsden, C.A. Cyclic mesoionic compounds. XIII. Mass spectra of mesoionic heterocycles. J. Chem. Soc. Perkin Trans. 1 1974, 6, 645–650. [Google Scholar] [CrossRef]
- Atkin, C.W.; Barnes, A.N.M.; Edgerley, P.G.; Sutton, L.E. Cyclic mesoionic compounds. VII. Electric dipole moments of some mesoionic 1,3,4-thiadiazoles. J. Chem. Soc. B 1969, 9, 1194–1196. [Google Scholar] [CrossRef]
- Baker, W.; Ollis, W.D. Meso-ionic compounds. Quart. Rev. 1957, 11, 15–29. [Google Scholar] [CrossRef]
- Kier, L.B.; Roche, E.B. Medicinal chemistry of the mesoionic compounds. J. Pharmaceut. Sci. 1967, 56, 149–168. [Google Scholar] [CrossRef]
- Ohta, N.; Kato, H.; Kaneko, T. Structure of busch’s endo-thiatriazolines. Bull. Chem. Soc. Jap. 1967, 40, 579–583. [Google Scholar] [CrossRef]
- Grashey, R.; Baumann, M.; Lubos, W.-D. Mesoionische 1,3,4-Thiadiazol-2-thione. Tetrahedron Lett. 1968, 56, 5881–5884. [Google Scholar] [CrossRef]
- Kier, L.B.; Scott, M.K. The synthesis of dialkyl mesoionic 1,3,4-thiadiazoles. J. Heterocycl. Chem. 1968, 5, 277–279. [Google Scholar] [CrossRef]
- Levi, T.G. Dithioformic acid II. Atti Accad. Naz. Lincei 1929, 9, 170–175. [Google Scholar]
- Gattow, G.; Dräger, M.; Engler, R. Über Dithioformiate. Naturwissenschaften 1971, 58, 53. [Google Scholar]
- Engler, R.; Gattow, G.; Dräger, M. Untersuchungen über Thioameisensäuren. 2. Darstellung und Eigenschaften von Dithioformiaten. Z. Anorg. Allg. Chem. 1972, 388, 229–237. [Google Scholar] [CrossRef]
- Martin, K. Potassium dithioformiate synthesis. Chem. Br. 1988, 24, 427–428. [Google Scholar]
- Urben, P.G.; Pitt, M.J. (Eds.) . Bretherick’s Handbook of Reactive Chemical Hazards, 6th ed.; Butterworth Heinemann: Oxford, UK, 1999; Volume 1, pp. 145–146. [Google Scholar]
- Muraoka, M.; Yamamoto, T.; Enomoto, K.; Takeshima, T. Synthesis of 2-alkoxycarbonyl enamino thioaldehydes and selenaldehydes (as pentacarbonyltungsten (0) complexes). Improved synthesis of simple and 2-cyano enamino thioaldehydes and some chemical reactions of these compounds. J. Chem. Soc. Perkin Trans. 1 1989, 7, 1241–1252. [Google Scholar] [CrossRef]
- Schauer, S.J.; Eyman, D.P.; Bernhardt, R.J.; Wolff, M.A.; Mallis, L.M. Synthesis and reactivity of (ŋ6-C6H6)Mn(CO)2SC(S)H and (ŋ6-C6(CH3)6)Mn(CO)2SC(S)H. Inorg. Chem. 1991, 30, 570–572. [Google Scholar] [CrossRef]
- Binder, H.; Diamantikos, W. Die Reaktion zwischen Schwefel bzw. Kohlenstoflfdisulfid und Natriumboranat in Aminen. Zwei neue Darstellungsmethoden für Alkylaminborane, R3-nHnN-BH3. Z. Naturforsch. 1983, 38b, 203–207. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, J.; Xin, M.; Jiang, Y.; Du, Z.; Lu, G.; Jiang, J.; Chan, A.S.C.; Ke, Z.; Zou, Y. Chalcone-based synthesis of tetrahydropyridazines via cloke–wilson-type rearrangement-involved tandem reaction between cyclopropyl ketones and hydrazines. J. Org. Chem. 2024, 89, 2726–2740. [Google Scholar] [CrossRef]
- Baker, W.; Ollis, W.D.; Phillips, A.; Strawford, T. Cyclic meso-ionic compounds. Part IV. ψ-4-Aryl-2: 4-dihydro-2-thio-1-thia-3: 4-diazoles (“endothiodihydrothiodiazoles”). J. Chem. Soc. 1951, 0, 289–291. [Google Scholar] [CrossRef]
- Guo, H.M.; Wang, J.J.; Xiong, Y.; Wu, X. Visible-light-driven multicomponent reactions for the versatile synthesis of thioamides by radical thiocarbamoylation. Angew. Chem. Int. Ed. 2024, 63, e202409605. [Google Scholar] [CrossRef]
- Li, E.; Hu, Z.; Song, L.; Yu, W.; Chang, J. Synthesis of 1,2,4-triazolo[4,3-a]pyridines and related heterocycles by sequential condensation and iodine-mediated oxidative cyclization. Chem. Eur. J. 2016, 22, 11022–11027. [Google Scholar] [CrossRef]
- Fargher, R.G.; Furness, R. Derivatives of 2-pyridylhydrazine and 2-quinolylhydrazine. J. Chem. Soc. Perkin Trans. 1 1915, 107, 688–699. [Google Scholar] [CrossRef]
- Tarbell, D.S.; Todd, C.W.; Paulson, M.C.; Lindstrom, E.G.; Wystrach, V.P. Synthesis of some substituted thiocarbazones. J. Am. Chem. Soc. 1948, 70, 1381–1385. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Ahamedzade, M.; Kirilmis, C.; Cukurovali, A.; Dilsiz, N. Synthesis and antimicrobial activity of new thiazole-2H(3H)-thiones containing 1,1,3-trisubstituted cyclobutene. S. Afr. J. Chem. 2003, 56, 21–24. [Google Scholar]
- Cambi, L.; Bargigia, G.; Paglia, E.D.; Ricca, G.S. Sui prodotti di riduzione dei tetrazo-tritioderivati dagli acidi ditiocarbazici. Atti Della Accad. Naz. Dei Lincei. Cl. Di Sci. Fis. Mat. E Naturali. Rend. 1968, 45, 330–338. [Google Scholar]
- Busch, M. Über heterobicyklische Verbindungen der Thiobiazol- und Triazolreihe. J. prakt. Chem. 1903, 67, 201–215. [Google Scholar] [CrossRef]
- Cmoch, P.; Stefaniak, L.; Melzer, E.; Bałoniak, S.; Webb, G.A. 1H, 13C and 15N NMR study of some triazolo- and tetrazolopyridazines and thioxotriazolopyridines. Magn. Reson. Chem. 1999, 37, 493–497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).