An Alternative Method for Preparing Methyl 2-Ferrocenyl-2-oxo-acetate
Abstract
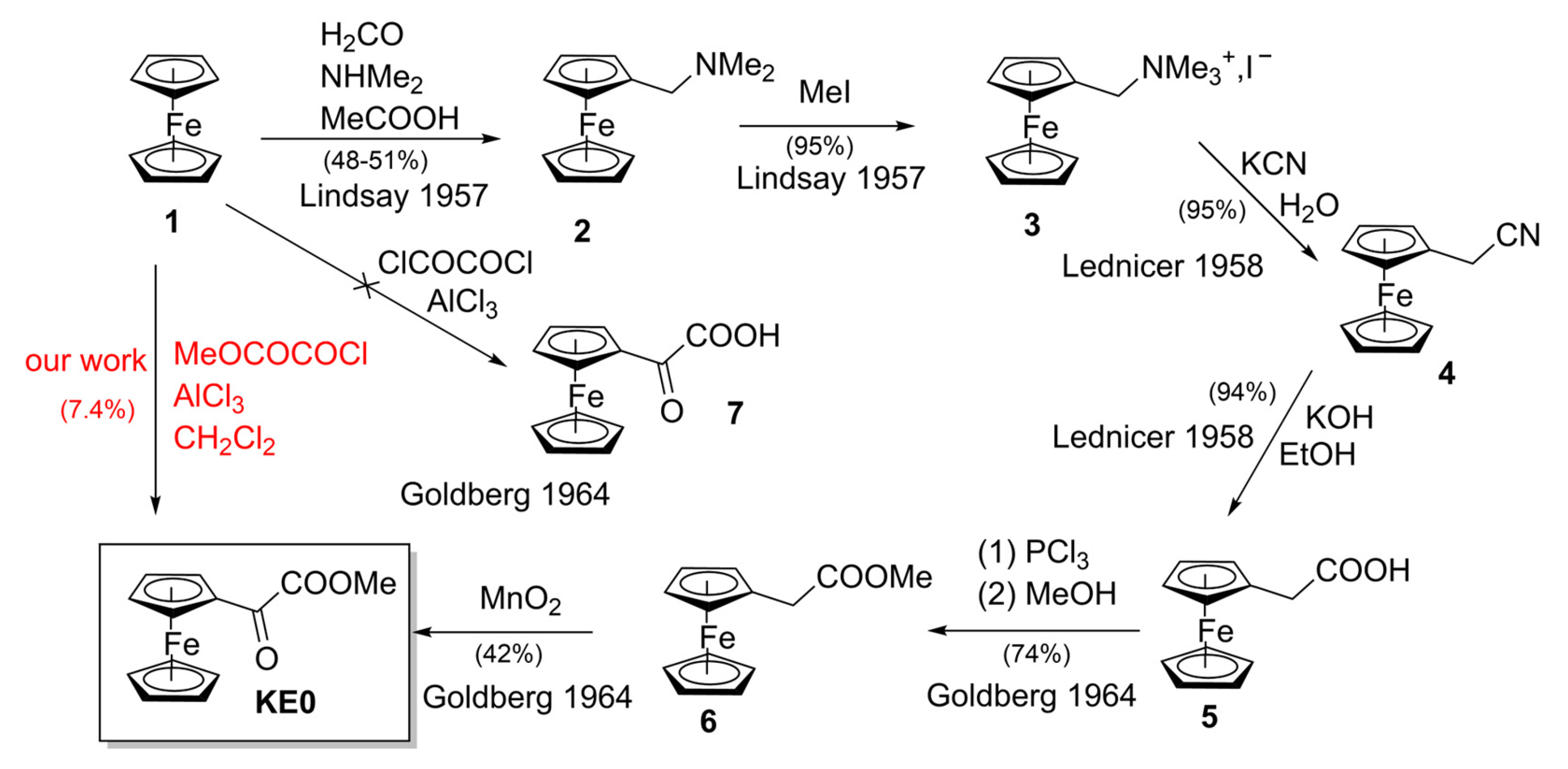
1. Introduction
2. Results and Discussion
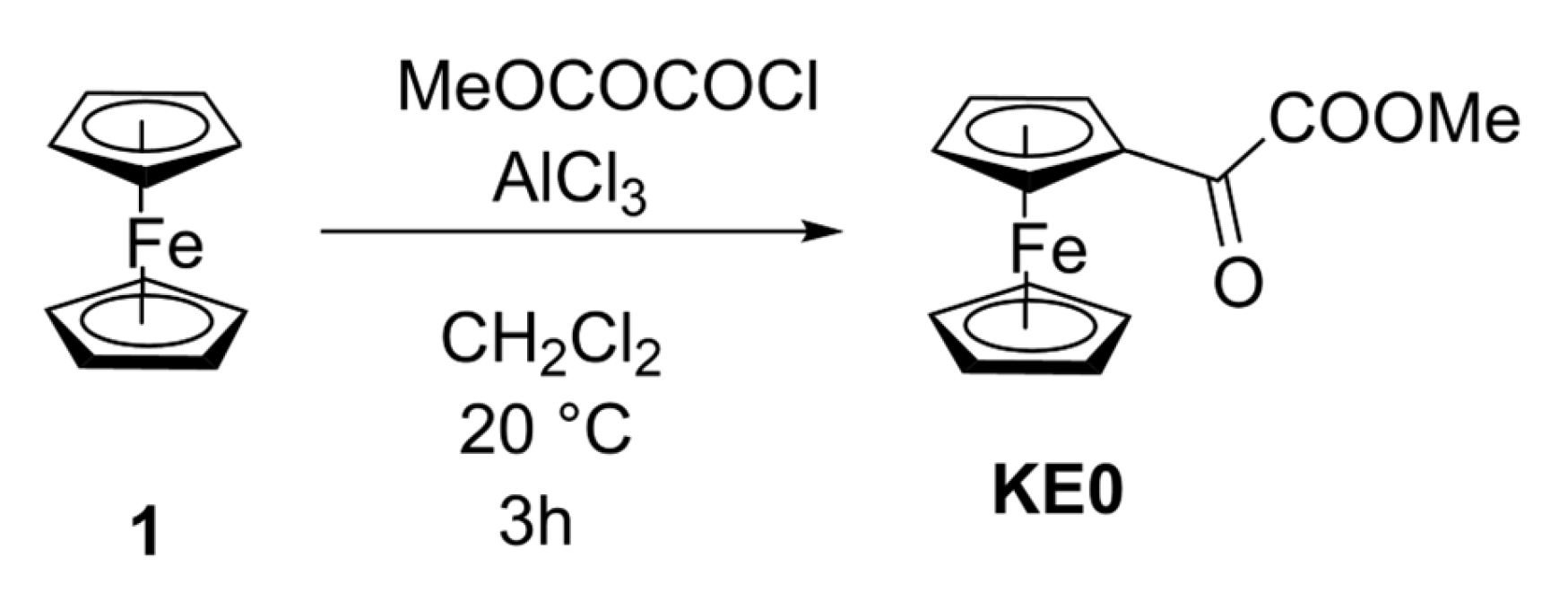
2.1. Synthesis of KE0 and Study of the Conditions
2.2. Characterization of KE0
3. Materials and Methods
3.1. General Procedure
3.2. Synthesis of Methyl 2-Ferrocenyl-2-oxo-acetate KE0
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauf, U.; Shabir, G.; Bukhari, S.; Albericio, F.; Saeed, A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules 2023, 28, 5765. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Astruc, D. Electron-reservoir applications of ferrocenes and other late transition-metal sandwich complexes: Flow batteries, sensing, catalysis, and biomedicine. Coord. Chem. Rev. 2025, 524, 216300. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti-cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The Medicinal Chemistry of Ferrocene and Its Derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Tomar, V.; Kumar, P.; Sharma, D.; Joshi, R.K.; Nemiwal, M. Anticancer potential of ferrocene-containing derivatives: Current and future prospective. J. Mol. Struct. 2025, 1319, 139589. [Google Scholar] [CrossRef]
- Tzeliou, C.E.; Zois, K.P.; Tzeli, D. Molecular Logic Gates Based on Ferrocene-Containing Compounds. Inorganics 2024, 12, 106. [Google Scholar] [CrossRef]
- Valdebenito, C.; Gaete, J.; Osorio, C.; Dibdalli, Y.; Norambuena, A.; Lecaros, N.; Carrasco, C.; Reyes, H.; Abarca, G.; Morales-Verdejo, C. Evaluation of Mono and Bimetallic Ferrocene-Based 1,2,3-Triazolyl Compounds as Burning Rate Catalysts for Solid Rocket Motor. ACS Omega 2023, 8, 35242–35255. [Google Scholar] [CrossRef] [PubMed]
- Carty, P.; Grant, J.; Simpson, A. Synthesis of a novel ferrocene derivative having flame-retardant and smoke-suppressant properties. Appl. Organomet. Chem. 1988, 2, 277–280. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Xie, P.-P.; Yang, S.; Pigeon, P.; Xiong, Y.; Gai, S.; Qi, X.; Wang, J.; Zhang, Q.; et al. Deciphering the Diversified Metabolic Behavior of Hydroxyalkyl Ferrocidiphenols as Anticancer Complexes. J. Med. Chem. 2023, 67, 1209–1224. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. Int. Ed. 2015, 54, 10230–10233. [Google Scholar] [CrossRef]
- Goldberg, S.I.; Keith, L.H. Synthesis of Methoxalylferrocene. J. Chem. Eng. Data 1964, 9, 250–251. [Google Scholar] [CrossRef]
- Lindsay, J.K.; Hauser, C.R. Aminomethylation of ferrocene to form N,N-dimethylaminomethylferrocene and its conversion to the corresponding alcohol and aldehyde. J. Org. Chem. 1957, 22, 355–358. [Google Scholar] [CrossRef]
- Pigeon, P.; Gaschard, M.; Othman, M.; Salmain, M.; Jaouen, G. α-Hydroxylactams as Efficient Entries to Diversely Functionalized Ferrociphenols: Synthesis and Antiproliferative Activity Studies. Molecules 2022, 27, 4549. [Google Scholar] [CrossRef]
- Lednicer, D.; Lindsay, J.K.; Hauser, C.R. Reaction of the methiodide of N,N-dimethylaminomethylferrocene with potassium cyanide to form ferrocylacetonitrile. J. Org. Chem. 1958, 23, 653–655. [Google Scholar] [CrossRef]
- Ge, F.; Ye, H.; Luo, J.-Z.; Wang, S.; Sun, Y.-J.; Zhao, B.-X.; Miao, J.-Y. A new fluorescent and colorimetric chemosensor for Cu(II) based on rhodamine hydrazone and ferrocene unit. Sens. Actuators B Chem. 2013, 181, 215–220. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Gu, Z. BF3·OEt2-Promoted Synthesis of 2,3-Metallocenocyclohexanones: A 1,2-Hydride Shift and Cationic Cyclization Strategy. J. Org. Chem. 2015, 80, 7865–7875. [Google Scholar] [CrossRef]
- Zherebker, K.Y.; Rodionov, A.N.; Pilipenko, E.S.; Kachala, V.V.; Nikitin, O.M.; Belousov, Y.A.; Simenel, A.A. The synthesis of ferrocenyl- and ferrocenoylpyrimidines. Russ. J. Org. Chem. 2014, 50, 1150–1154. [Google Scholar] [CrossRef]
- Lee, B.C.; Choe, Y.S.; Chi, D.Y.; Paik, J.-Y.; Lee, K.-H.; Choi, Y.; Kim, B.-T. 8-Cyclopentadienyltricarbonyl 99mTc 8-Oxooctanoic Acid: A Novel Radiotracer for Evaluation of Medium Chain Fatty Acid Metabolism in the Liver. Bioconjugate Chem. 2004, 15, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Tachi, M. Effect of the ferrocene nucleus on the microwave-accelerated esterification reaction. Int. J. Chem. 2023, 15, 26–33. [Google Scholar] [CrossRef]
- Wieczorek, A.; Blauz, A.; Makal, A.; Rychlik, B.; Plazuk, D. Synthesis and evaluation of biological properties of ferrocenyl-podophyllotoxin conjugates. Dalton Trans. 2017, 46, 10847–10858. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H. Synthesis and biological evaluation of fatty acids conjugates bearing cyclopentadienyl-donors incorporated [99mTc/Re(CO)3]+ for myocardial imaging. Eur. J. Med. Chem. 2014, 72, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wilbert, G.; Traud, S.; Zentel, R. Liquid crystalline main-chain polymers containing the ferrocene unit as a side group, 2. Variation of the spacer length. Macromol. Chem. Phys. 1997, 198, 3769–3785. [Google Scholar] [CrossRef]
- Uehara, T.; Uemura, T.; Hirabayashi, S.; Adachi, S.; Odaka, K.; Akizawa, H.; Magata, Y.; Irie, T.; Arano, Y. Technetium-99m-Labeled Long Chain Fatty Acid Analogues Metabolized by β-Oxidation in the Heart. J. Med. Chem. 2007, 50, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Seiders, R.P.; Brookhart, M.; Whitten, D.G. Synthesis and reactivity of a surfactant ferrocene. Environmental effects on oxidation of ferrocene in organized media. Isr. J. Chem. 1980, 18, 272–278. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, D.H.; Lee, I.; Choe, Y.S.; Chi, D.Y.; Lee, K.-H.; Choi, Y.; Kim, B.-T. 16-Cyclopentadienyl Tricarbonyl 99mTc 16-Oxo-hexadecanoic Acid: Synthesis and Evaluation of Fatty Acid Metabolism in Mouse Myocardium. J. Med. Chem. 2008, 51, 3630–3634. [Google Scholar] [CrossRef]
- Turbitt, T.D.; Watts, W.E. Bridged ferrocenes: XII. The synthesis of [3] ferrocenophan-1-one from ferrocene by a novel one-step annelation reaction. Organomet. Chem. 1972, 46, 109–117. [Google Scholar] [CrossRef]
- Görmen, M.; Pigeon, P.; Top, S.; Hillard, E.A.; Huché, M.; Hartinger, C.G.; de Montigny, F.; Plamont, M.-A.; Vessières, A.; Jaouen, G. Synthesis, Cytotoxicity, and COMPARE Analysis of Ferrocene and [3]Ferrocenophane Tetrasubstituted Olefin Derivatives against Human Cancer Cells. ChemMedChem 2010, 5, 2039–2050. [Google Scholar] [CrossRef]
 | |||||||
|---|---|---|---|---|---|---|---|
| Number | Ref. | Number | Ref. | Number | Ref. | Number | Ref. |
| KE0 | [11] | KE1 | [15,16,17] | KE2 | [18] | KE3 | [19] |
| KE4 | [18,20,21] | KE5 | Et | KE6 | [18] | KE7 | Pat. |
| KE8 | [22] | KE9 | Pat. | KE10 | Et | KE11 | Et |
| KE12 | n.f. | KE13 | [21,23] | KE14 | [24,25] | KE15 | n.f. |
| Entry | T | Time (h) | AlCl3 (eq.) b,c | CH2Cl2 (mL) | Yield c (%) |
|---|---|---|---|---|---|
| 1 | 0–5 °C | 2 | 1.5 | 60 | 4.0 |
| 2 | r.t. a | 2 | 1.5 | 60 | 4.3 |
| 3 | r.t. | 1 | 1.5 | 60 | 2.7 |
| 4 | r.t. | 3 | 1.5 | 60 | 7.4 |
| 5 | r.t. | 4 | 1.5 | 60 | 4.1 |
| 6 | r.t. | 3 | 1.5 | 180 | 2.9 |
| 7 | r.t. | 3 | 1.0 | 60 | 3.3 |
| 8 | r.t. | 3 | 2.0 | 60 | 1.3 |
| 9 | −68 °C | 24 | 1.5 | 60 | 1.2 |
| 10 | −29 °C | 24 | 1.5 | 60 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigeon, P.; Hapel, H. An Alternative Method for Preparing Methyl 2-Ferrocenyl-2-oxo-acetate. Molbank 2025, 2025, M2009. https://doi.org/10.3390/M2009
Pigeon P, Hapel H. An Alternative Method for Preparing Methyl 2-Ferrocenyl-2-oxo-acetate. Molbank. 2025; 2025(2):M2009. https://doi.org/10.3390/M2009
Chicago/Turabian StylePigeon, Pascal, and Hugo Hapel. 2025. "An Alternative Method for Preparing Methyl 2-Ferrocenyl-2-oxo-acetate" Molbank 2025, no. 2: M2009. https://doi.org/10.3390/M2009
APA StylePigeon, P., & Hapel, H. (2025). An Alternative Method for Preparing Methyl 2-Ferrocenyl-2-oxo-acetate. Molbank, 2025(2), M2009. https://doi.org/10.3390/M2009







