Abstract
Representatives of the hexasubstituted benzene derivatives, also known as hexa-hosts, have been the subject of extensive studies in solution and in the solid state, including the investigation of their ability to act as artificial receptors for various substrates, as well as detailed conformational analyses. In this paper, we describe the X-ray crystal structure of hexakis(acetoxymethyl)benzene (1), a member of the above class of compounds. The molecules of 1 adopt an aaabbb conformation, in which three side-arms point to the same face of the central benzene ring, while the other three point in the opposite direction. As the compound lacks strong hydrogen bond donors, C–H···O hydrogen bonds connect the molecules to a three-dimensional supramolecular network. According to the Hirshfeld surface analysis, the H∙∙∙O/O∙∙∙H interactions represent the major contribution of the molecular Hirshfeld surface.
1. Introduction
Hexasubstituted benzene derivatives, in their function as hexa-hosts [1,2,3], were shown to have interesting binding properties towards various substrates [4,5,6,7,8,9,10]. The potential of these compounds to act as artificial receptors was evaluated both in the solution and in the solid state. Analyses of the conformations of various hexasubstituted benzenes [6,7,8,9,10,11,12,13,14,15] revealed that the ababab conformation, with an alternating arrangement of the side-arms above (a) and below (b) the plane of the benzene ring, is frequently observed in crystal structures. In addition, such conformations as aaabbb, aaabab, and even aaaaaa have also been observed. In the case of hexakis(acetoxymethyl)benzene (1; see Figure 1), the crystal structure of which is described in this paper, the molecules adopt an aaabbb conformation.
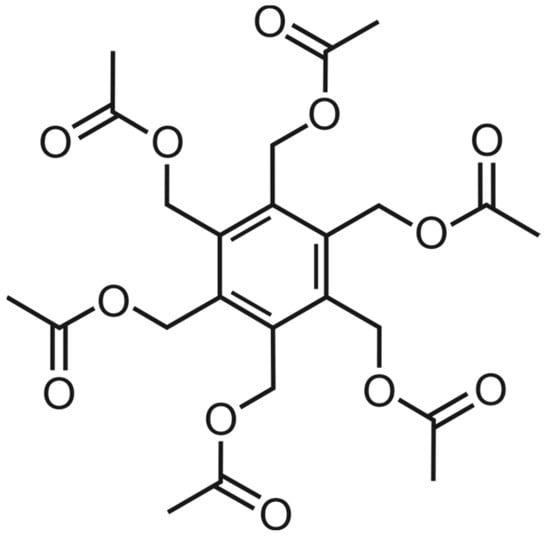
Figure 1.
Molecular structure of compound 1.
Since compound 1 has no strong hydrogen bond donors, the crystal is stabilized by numerous C–H···O hydrogen bonds. It should be noted that the C–H···O interaction has long been the subject of controversial discussions. Intensive studies revealed its hydrogen bonding character and its important role in crystal packing [16,17,18,19,20,21,22] and in various recognition processes in artificial and biological systems. There are many examples of the involvement of C–H···O hydrogen bonds in such processes [23,24,25,26,27], ranging from the molecular recognition of carbohydrates by artificial receptors [25] to protein–ligand interactions [26] and participation in the interactions of adjacent strands of parallel and antiparallel β-sheets in proteins [27].
2. Results and Discussion
2.1. Synthesis
Hexakis(acetoxymethyl)benzene (1) was synthesized according to Backer’s procedure [28], in which hexakis(bromomethyl)benzene (4) is reacted with potassium acetate in acetic anhydride. The synthesis of compound 4 started with the bromomethylation of mesitylene (2) to give 1,3,5-tris(bromomethyl)-2,4,6-trimethylbenzene (3), followed by the bromination of the methyl groups of 3 [29]. All reaction steps are summarized in Scheme 1.
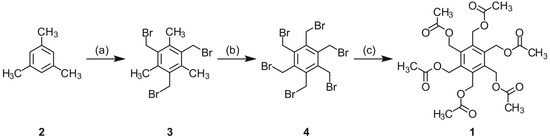
Scheme 1.
Synthesis of compound 1 (according to procedures in the literature [28,29]). Reagents and conditions: (a) (CH2O)n, HBr/CH3COOH, ZnBr2, 14 h, 95 °C (97% of 3); (b) Br2, 1,2-dibromoethane, 15 h, reflux (80% of 4); (c) potassium acetate, acetic anhydride, 4 h, reflux (72% of 1).
The formation of hexakis(acetoxymethyl)benzene (1) has also been described for the reaction of diisopropylacetylene and 1,4-diacetoxy-2-butyne in the presence of Hg[Co(CO)4]2, yielding a mixture of products, including compound 1 (formed by the trimerization of 1,4-diacetoxy-2-butyne) [30,31].
2.2. Structure Elucidation
The crystal structure of the hexapodal compound 1, the molecular structure of which is shown in Figure 2a, was solved in the monoclinic space group P21/c (Table S1 in Supporting Information). The asymmetric unit of the elementary cell contains one complete molecule and one half of the molecule, i.e., one of the molecules is found in a general position, whereas the second one is located on a crystallographic symmetry center. Nevertheless, both molecules adopt similar geometries with a three-up/three-down orientation (aaabbb pattern) of the substituents around the perimeter of the aromatic ring. The torsion angles of the Caryl–C–O–C sequences in molecule A range from 130.6(1) to 179.7(1)°, while those of molecule B are 120.1(1)–175.7(1)° (Table S2 in Supporting Information). In molecule A, two of the substituents exist in an elongated conformation, while the other four show a high degree of twisting. In the case of the inversion-symmetric molecule (B), four of the branches adopt a fully extended conformation, while the remaining ones are considerably twisted. These conformational differences are also obvious from the superposition of the independent molecules displayed in Figure 2b. As can be seen from Table S3 (Supporting Information), the crystal is stabilized by a variety of intermolecular C–H···O hydrogen bonds [16] [d(H···O) 2.41–2.57 Å, ∢(C–H···O) 119.7–171.2°]. With the exception of O10, all carbonyl oxygen atoms are involved in molecular association, either by acting as single or bifurcated acceptors. A packing excerpt showing the mode of intermolecular interactions is depicted in Figure 3a. The scheme shown in Figure 3b represents a part of the C–H···O bonds occurring in the crystal structure, in which the methylene hydrogen atoms are primarily involved due to packing effects. However, it should be noted that the more acidic methyl hydrogen atoms also contribute, but to a lesser extent, to the formation of the hydrogen bonding pattern.
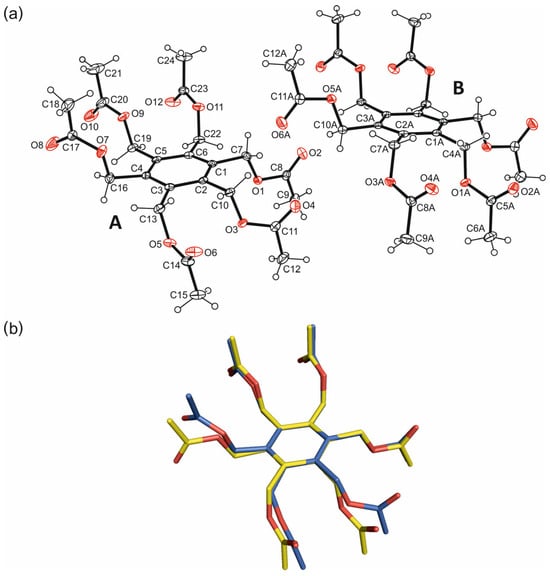
Figure 2.
(a) Perspective view of the molecular structure of hexakis(acetoxymethyl)benzene including atom numbering. Displacement ellipsoids are drawn at a 50% probability level. (b) Superposition of the molecular structures A (blue) and B (yellow); oxygen atoms are marked in red.
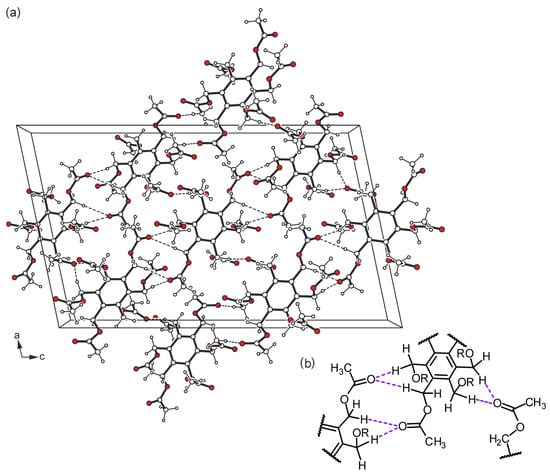
Figure 3.
(a) Packing diagram of 1 viewed along the crystallographic b axis. Dashed lines represent hydrogen bonds. (b) Schematic representation of the mode of hydrogen bonding [R = −(C=O)CH3].
2.3. Hirshfeld Surface Analysis
To visualize and quantify the intermolecular contacts present in the crystal structure, the CrystalExplorer 17.5 software [32] was used to generate the Hirshfeld surfaces [33] and the associated two-dimensional fingerprint plots [34] of the independent molecules. At this point, it should be noted that the standard C–H bond lengths given by SHELXL are biased by 0.1–0.15 Å and are replaced here by a more realistic value of 1.083 Å.
In the Hirshfeld surface plotted over dnorm, the blue-, white- and red-colored regions represent intermolecular contacts with distances longer than, equal to, or shorter than the sum of the van der Waals radii [35] of the atoms. The bright red spots on the dnorm surfaces shown in Figure 4 arise from short H∙∙∙O contacts, resulting from C–H···O bonds. They are visible in the fingerprint plots of the molecules as a pair of symmetrical short spikes with a minimum value of de + di ≈ 2.35 Å (see Figure S1, Supporting Information). The decomposition of the 2D fingerprint plots reveals that H∙∙∙O/O∙∙∙H interactions represent the main contribution (49.2 and 48.3%) of the molecular Hirshfeld surface (see Figure 5). H∙∙∙H contacts, which correspond to van der Waals interactions, appear as the next largest region, making up 39.6 and 41.4% of the Hirshfeld surfaces. They can be recognized in the fingerprint plots as a region of widely scattered points with a closest distance of de + di ≈ 2.24 Å. The H∙∙∙C/C∙∙∙H interactions cover a range of 10.2 and 9.1%.
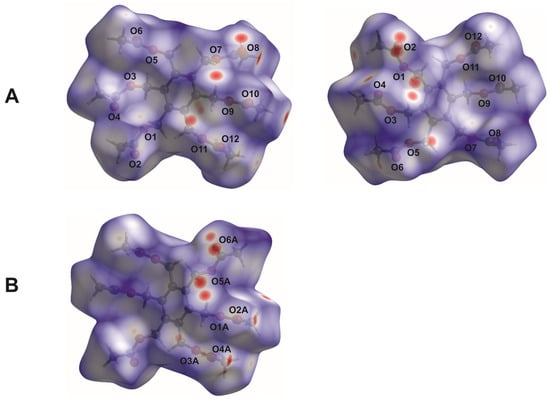
Figure 4.
View of the Hirshfeld surfaces of the molecules mapped over dnorm in the range of −0.1811 to 1.2749 (arbitrary units). The capital letters A and B assign the Hirshfeld surfaces to the different molecular structures (see Figure 2a). For molecule A, two sides are displayed.
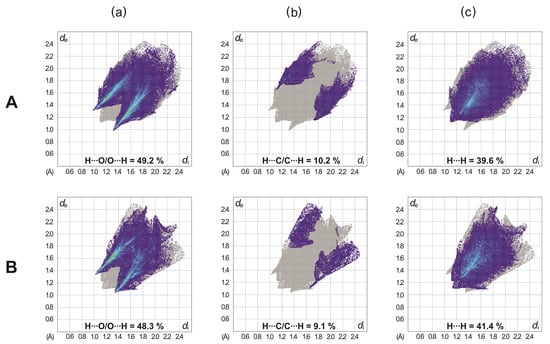
Figure 5.
Two-dimensional fingerprint plots of the independent molecules delineated into (a) H∙∙∙O/O∙∙∙H, (b) H∙∙∙C/C∙∙∙H, and (c) H∙∙∙H interactions (plots for all intermolecular interactions are shown in Figure S1, Supporting Information). The capital letters A and B assign the fingerprint plots to the different molecular structures (see Figure 2a).
3. Materials and Methods
3.1. Synthesis
Hexakis(bromomethyl)benzene (4; 2.00 g, 3.15 mmol) and potassium acetate (3.13 g, 31.89 mmol) were suspended in acetic anhydride (25 mL) and stirred under reflux for 4 h. The reaction mixture was poured into water (350 mL), forming a brown precipitate. After stirring at room temperature for 2 h, the solid was filtered off and washed with water. Recrystallization of the crude product from ethanol yielded colorless crystals of 1 (1.16 g, 72%). M.p. 159–160 °C. TLC: Rf = 0.21 [SiO2; eluent: toluene/ethyl acetate 3:1 v/v]. 1H NMR (500 MHz, CDCl3, ppm): δ = 2.06 (s, 18 H, CH3), 5.38 (s, 12 H, CH2). 13C NMR (125 MHz, CDCl3, ppm): δ = 20.9 (CH3), 60.0 (CH2), 137.6 (CAr), 170.3 (C=O). LC-MS (ESI) m/z calcd. for C24H30O12Na: 533.16 [M + Na]+, found 533.13. Anal. calc. for C24H30O12: C, 56.47; H, 5.92%; found: C, 56.41; H, 5.82%.
The synthesis of the starting material, hexakis(bromomethyl)benzene (4), was carried out according to the literature [29].
3.2. Crystallization and X-Ray Structure Determination
Crystals of the compound were grown by the slow evaporation of toluene from a corresponding solution at room temperature. The crystal suitable for the X-ray diffraction experiment was selected under the microscope and mounted on a glass fiber. The intensity data were collected on a Bruker APEX II diffractometer [36] with MoKα radiation (λ = 0.71073 Å) using ω and φ scans. Data integration and reduction were processed with SAINT [36]. Absorption correction was carried out by using SADABS [37]. Preliminary structure models were derived by the application of direct methods [38] and refined by full-matrix least-squares calculation based on F2 for all reflections [39]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were included in the models in calculated positions and were refined as constrained to bonding atoms. The crystallographic data are given below and in the Supplementary Materials (Table S1).
Crystal data for C24H30O12 (M = 510.48 g/mol): monoclinic; space group P21/c (no. 14); a = 16.6400(7) Å; b = 8.1525(3) Å; c = 27.577(1) Å; β = 101.902(1)°; V = 3660.6(2) Å3; Z = 6; T = 100(2) K; µ(MoKα) = 0.112 mm−1; Dcalc = 1.389 g/cm3; 8914 reflections measured (1.5° ≤ θ ≤ 28.1°); 8202 unique reflections (Rint = 0.0184), which were used in all calculations. The final R1 was 0.0379 (I > 2σ(I)) and wR2 was 0.1029 (all data), with GOF = 1.019.
4. Conclusions
This paper describes the X-ray crystal structure of hexakis(acetoxymethyl)benzene (1), a representative of the class of hexasubstituted benzenes (some of them also known as hexa-hosts). The crystals of 1 obtained from toluene have the monoclinic space group P21/c and the molecules adopt a conformation in which the side-arms are arranged in aaabbb fashion around the central benzene ring. A large number of intermolecular C–H···O hydrogen bonds are responsible for the stabilization of the crystal structure. The carbonyl oxygen atoms of 1 are involved in these hydrogen bonding interactions, either by acting as single or bifurcated acceptors. As revealed by Hirshfeld surface analysis, the H∙∙∙O/O∙∙∙H interactions represent the largest contribution (49.2 and 48.3%) of the molecular Hirshfeld surface.
Supplementary Materials
The following supporting information are available online. Figure S1: Two-dimensional fingerprint plots of the independent molecules showing all intermolecular interactions; Figure S2 and Figure S3: 1H and 13C NMR spectra of 1 in CDCl3; Table S1: Crystallographic data and refinement parameters of the compound studied; Table S2: Selected torsion angles; Table S3: Geometric parameters for hydrogen bond interactions in the crystal structure; general experimental details.
Author Contributions
Conceptualization, M.M.; investigation, M.S.; writing—original draft preparation, W.S. and M.M.; writing—review and editing, M.S. and M.M.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2442747 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/? (or from the CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK; e-mail: deposit@ccdc.cam.ac.uk).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- MacNicol, D.D.; Hardy, A.D.U.; Wilson, D.R. Crystal and molecular structure of a ‘hexa-host’ inclusion compound. Nature 1977, 266, 611–612. [Google Scholar] [CrossRef]
- MacNicol, D.D.; Wilson, D.R. New Strategy for the Design of Inclusion Compounds: Discovery of the ‘Hexa-hosts’. J. Chem. Soc. Chem. Commun. 1976, 494–495. [Google Scholar] [CrossRef]
- Vögtle, F.; Weber, E. Krakenmoleküle. Angew. Chem. 1974, 86, 896–898. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ahamed, B.N.; Ghosh, P. Binding of Ammonium Hexafluorophosphate and Cation-Induced Isolation of Unusual Conformers of a Hexapodal Receptor. Org. Lett. 2010, 12, 2742–2745. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Ding, W.; Chen, X.; Wu, L.; Huang, Z.; Zhang, Z. Pyrrole-based acyclic hexapodal aldehyde for anion recognition. Tetrahedron 2024, 165, 134170. [Google Scholar] [CrossRef]
- Koch, N.; Seichter, W.; Mazik, M. Hexapodal pyrazole-based receptors: Complexes with ammonium ions and solvent molecules in the solid state. Tetrahedron 2015, 71, 8965–8974. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ghosh, P. Bistripodand Amide Host for Compartmental Recognition of Multiple Oxyanions. Org. Lett. 2010, 12, 328–331. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, R.; Wong, B.M.; Ghosh, P. Anion directed conformational diversities of an arene based hexa-amide receptor and recognition of the [F4(H2O)6]4− cluster. RSC Adv. 2014, 4, 62689–62693. [Google Scholar] [CrossRef]
- Arunachalam, M.; Ghosh, P. Encapsulation of [F4(H2O)10]4− in a dimeric assembly of an unidirectional arene based hexapodal amide receptor. Chem. Commun. 2011, 22, 6269–6271. [Google Scholar] [CrossRef]
- Chakraborty, S.; Arunachalam, M.; Dutta, R.; Ghosh, P. Arene platform based hexa-amide receptors for anion recognition: Single crystal X-ray structural and thermodynamic studies. RSC Adv. 2015, 5, 48060–48070. [Google Scholar] [CrossRef]
- Förster, S.; Seichter, W.; Weber, E. Synthesis and Structures of Three- and Hexa-armed Benzene Derivatives Featuring Lateral Benzoic Ester and Benzoic Acid Functions. Z. Naturforschung B 2011, 66, 939–946. [Google Scholar] [CrossRef]
- Das, D.; Barbour, L.J. Polymorphism of a Hexa-host: Isolation of Four Different Single-Crystal Phases by Melt Crystallization. J. Am. Chem. Soc. 2008, 130, 14032–14033. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Barbour, L.J. Unusual Conformations of a Hexa-Host Molecule in Solvate Inclusion Compounds. Cryst. Growth Des. 2009, 9, 1599–1604. [Google Scholar] [CrossRef]
- Das, D.; Barbour, L.J. Concomitant formation of two different solvates of a hexa-host from a binary mixture of solvents. Chem. Commun. 2008, 5110–5112. [Google Scholar] [CrossRef]
- Gavette, J.V.; Sargent, A.L.; Allen, W.E. Hydrogen Bonding vs Steric Gearing in a Hexasubstituted Benzene. J. Org. Chem. 2008, 73, 3582–3584. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Steiner, T. Unrolling the hydrogen bond properties of C–H···O interactions. Chem. Commun. 1997, 727–734. [Google Scholar] [CrossRef]
- Steiner, T. Effect of acceptor strength on C–H···O hydrogen bond lengths as revealed by and quantified from crystallographic data. J. Chem. Soc. Chem. Commun. 1994, 2341–2342. [Google Scholar] [CrossRef]
- Steiner, T.; Desiraju, G.R. Distinction between the weak hydrogen bond and the van der Waals interaction. Chem. Commun. 1998, 891–892. [Google Scholar] [CrossRef]
- Desiraju, G.R. C–H···O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 2995–3001. [Google Scholar] [CrossRef]
- Mazik, M.; Bläser, D.; Boese, R. Intermolecular CH···N/CH···O hydrogen bonds in the crystal structures of α,β-unsaturated ketones carrying a terminal pyridine subunit. Tetrahedron 2001, 57, 5791–5797. [Google Scholar] [CrossRef]
- Mazik, M.; Hartmann, A.; Jones, P.G. Hydrogen and Halogen Bonding in the Crystal Structure of a 1,3,5-Substituted 2,4,6-Triethylbenzene Consisting of Three Phenanthroline Units. Eur. J. Org. Chem. 2010, 2010, 458–463. [Google Scholar] [CrossRef]
- Castellano, R.K. Progress Toward Understanding the Nature and Function of C–H···O Interactions. Curr. Org. Chem. 2004, 8, 845–865. [Google Scholar] [CrossRef]
- Horowitz, S.; Trievel, R.C. Carbon-Oxygen Hydrogen Bonding in Biological Structure and Function. J. Biol. Chem. 2012, 287, 41576–41582. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, Y.; Jones, L.O.; Song, B.; Guo, Q.-H.; Zhang, L.; Qiu, Y.; Feng, Y.; Chen, X.-Y.; Schatz, G.C.; et al. PCage: Fluorescent Molecular Temples for Binding Sugars in Water. J. Am. Chem. Soc. 2021, 143, 15688–15700. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Nakashima, Y.; Tsukamoto, S.; Kurohara, T.; Suzuki, M.; Sakae, Y.; Oda, M.; Okamoto, Y.; Suzuki, T. N+–C–H···O Hydrogen bonds in protein-ligand complexes. Sci. Rep. 2019, 9, 767. [Google Scholar] [CrossRef]
- Scheiner, S. Weak H-bonds. Comparisons of CH···O to NH···O in proteins and PH···N to direct P···N interactions. Phys. Chem. Chem. Phys. 2011, 13, 13860–13872. [Google Scholar] [CrossRef]
- Backer, H.J. L’hexa-hydroxyméthyl-benzène et ses dérivés (composés planradiaires I). Recl. Trav. Chim. Pays-Bas 1935, 54, 833–837. [Google Scholar] [CrossRef]
- Závada, J.; Pánková, M.; Holý, P.; Tichý, M. A Facile Synthesis of Hexakis(bromomethyl)benzene from Mesitylene. Synthesis 1994, 1994, 1132. [Google Scholar] [CrossRef]
- Biali, S.E.; Mislow, K. Barrier to Internal Rotation in l,2-Bis(bromochloromethyl)-3,4,5,6-tetraisopropylbenzene. J. Org. Chem. 1988, 53, 1318–1320. [Google Scholar] [CrossRef]
- Biali, S.E.; Gutiérrez, A.; Mislow, K. Achiral Hexaisopropylbenzene Isotopomers: Analogues of the Achiral Trihydroxyglutaric Acid Diastereomers. J. Org. Chem. 1988, 53, 1316–1318. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Venkatesan, P.; Thamotharan, S.; Ilangovan, A.; Liang, H.; Sundius, T. Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino]prop-2-enoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 625–636. [Google Scholar] [CrossRef]
- APEX2 and SAINT. Bruker AXS Inc.: Madison, WI, USA, 2005.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).