Synthesis and Characterization of New Monosubstituted Pillar[5]arene with Terminal Carboxyl Group
Abstract
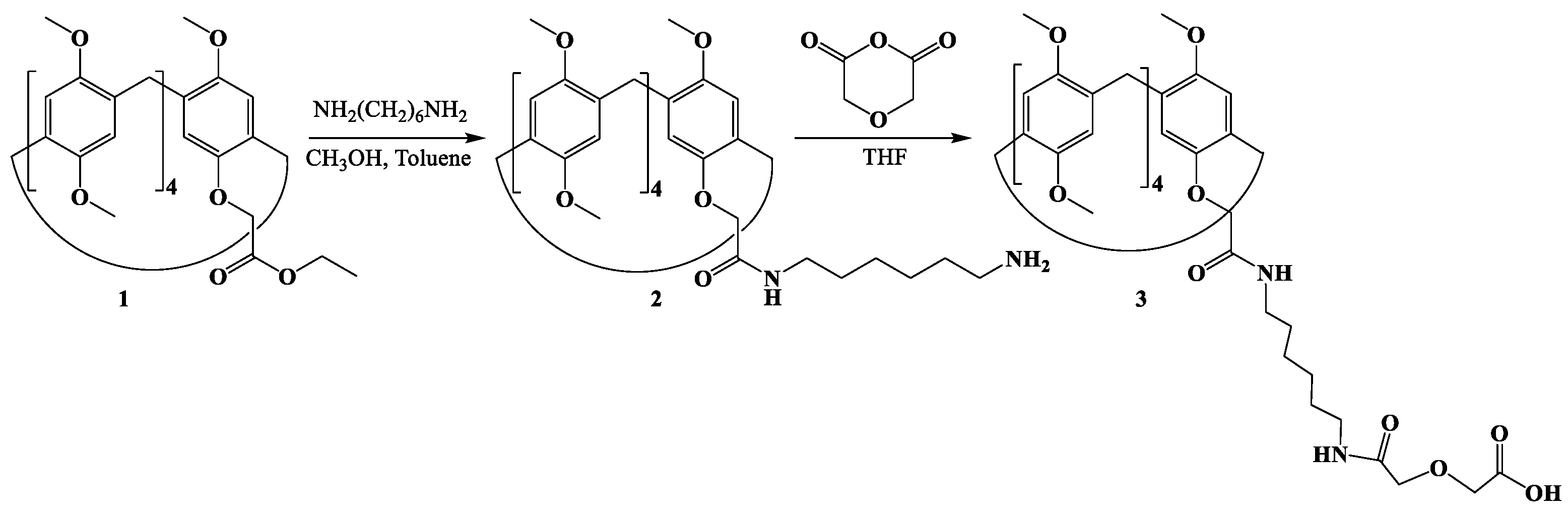
1. Introduction
2. Results and Discussion
3. Materials and Methods
General
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Rajoriya, V.; Gupta, R.; Vengurlekar, S.; Singh, U.S. Nanostructured lipid carriers (NLCs): A promising candidate for lung cancer targeting. Int. J. Pharm. 2024, 655, 123986. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Shengyi, W.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Yakimova, L.; Padnya, P.; Tereshina, D.; Kunafina, A.; Nugmanova, A.; Osin, Y.; Evtugyn, V.; Stoikov, I. Interpolyelectrolyte mixed nanoparticles from anionic and cationic thiacalix[4]arenes for selective recognition of model biopolymers. J. Mol. Liq. 2019, 279, 9–17. [Google Scholar] [CrossRef]
- Puris, E.; Fricker, G.; Gynther, M. The role of solute carrier transporters in efficient anticancer drug delivery and therapy. Pharmaceutics 2023, 15, 364. [Google Scholar] [CrossRef]
- Vishwakarma, M.; Agrawal, P.; Soni, S.; Tomar, S.; Haider, T.; Kashaw, S.K.; Soni, V. Cationic nanocarriers: A potential approach for targeting negatively charged cancer cell. Adv. Colloid Interface Sci. 2024, 327, 103160. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Ghaemi, A.; Khanizadeh, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Fathi-karkan, S. Targeted Nano-Delivery of Flutamide with polymeric and lipid nanoparticles. Eur. Polym. J. 2024, 213, 113124. [Google Scholar] [CrossRef]
- Cao, Z.; Zuo, X.; Liu, X.; Xu, G.; Yong, K.T. Recent progress in stimuli-responsive polymeric micelles for targeted delivery of functional nanoparticles. Adv. Colloid Interface Sci. 2024, 330, 103206. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Alzaidy, A.H.; Abed, M.J.; Rashki, S.; Salavati-Niasari, M. Advances in inorganic nanoparticles-based drug delivery in targeted breast cancer theranostics. Adv. Colloid Interface Sci. 2024, 329, 103204. [Google Scholar] [CrossRef]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-responsive and dual-targeting liposomes: From mechanisms to targeting strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid lipid nanoparticles vs. nanostructured lipid carriers: A comparative review. Pharmaceutics 2024, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Galukhin, A.; Erokhin, A.; Imatdinov, I.; Osin, Y. Investigation of DNA binding abilities of solid lipid nanoparticles based on p-tert-butylthiacalix[4]arene platform. RSC Adv. 2015, 5, 33351–33355. [Google Scholar] [CrossRef]
- Huang, Z.; Hua, S.; Yang, Y.; Fang, J. Development and evaluation of lipid nanoparticles for camptothecin delivery: A comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol. Sin. 2008, 29, 1094–1102. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, S.; Liu, C.; Zhang, H.; Zhao, Y. Pillar[n]arene-based polymeric systems for biomedical applications. Coord. Chem. Rev. 2023, 491, 215260. [Google Scholar] [CrossRef]
- Nazarova, A.; Yakimova, L.; Filimonova, D.; Stoikov, I. Surfactant effect on the physicochemical characteristics of solid lipid nanoparticles based on pillar[5]arenes. Int. J. Mol. Sci. 2022, 23, 779. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Guralnik, E.G.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Fluorescein-loaded solid lipid nanoparticles based on monoamine pillar[5]arene: Synthesis and interaction with DNA. ChemNanoMat 2018, 4, 919–923. [Google Scholar] [CrossRef]
- Li, X.; Shen, M.; Yang, J.; Liu, L.; Yang, Y.W. Pillararene-based stimuli-responsive supramolecular delivery systems for cancer therapy. Adv. Mater. 2024, 36, 2313317. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wang, S.; Dai, N.-N.; Xiao, Y.; Zhou, Y.; Tian, W.-C.; Sun, D.; Li, Q.; Wang, Y.; Wei, W.-T. Carbon-carbon triple bond cleavage and reconstitution to achieve aryl amidation using nitrous acid esters. Nat. Commun. 2025, 16, 993. [Google Scholar] [CrossRef]
- Han, Y.; Huo, G.-F.; Sun, J.; Yan, C.-G.; Lu, Y.; Lin, C.; Wang, L. Axle length- and solvent-controlled construction of (pseudo)[1]rotaxanes from mono-thiourea-functionalised pillar[5]arene derivatives. Supramol. Chem. 2017, 29, 547–552. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, D.; Wang, L.; He, M.; Zhou, L.; Shollmeyer, D.; Meier, H. Monoester copillar[5]arenes: Synthesis, unusual self-inclusion behavior, and molecular recognition. Chem.–Eur. J. 2013, 19, 7064–7070. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, A.; Padnya, P.; Cragg, P.J.; Stoikov, I. [1]Rotaxanes based on phosphorylated pillar[5]arenes. New J. Chem. 2022, 46, 2033–2037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimova, L.; Filimonova, D.; Nazarova, A.; Stoikov, I. Synthesis and Characterization of New Monosubstituted Pillar[5]arene with Terminal Carboxyl Group. Molbank 2025, 2025, M2004. https://doi.org/10.3390/M2004
Yakimova L, Filimonova D, Nazarova A, Stoikov I. Synthesis and Characterization of New Monosubstituted Pillar[5]arene with Terminal Carboxyl Group. Molbank. 2025; 2025(2):M2004. https://doi.org/10.3390/M2004
Chicago/Turabian StyleYakimova, Luidmila, Darya Filimonova, Anastasia Nazarova, and Ivan Stoikov. 2025. "Synthesis and Characterization of New Monosubstituted Pillar[5]arene with Terminal Carboxyl Group" Molbank 2025, no. 2: M2004. https://doi.org/10.3390/M2004
APA StyleYakimova, L., Filimonova, D., Nazarova, A., & Stoikov, I. (2025). Synthesis and Characterization of New Monosubstituted Pillar[5]arene with Terminal Carboxyl Group. Molbank, 2025(2), M2004. https://doi.org/10.3390/M2004







