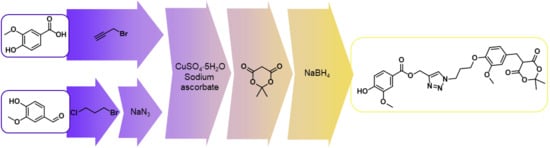
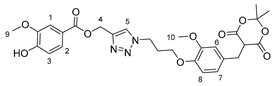
Synthesis of Vanillic Acid—Meldrum’s Acid Conjugate
Abstract
1. Introduction
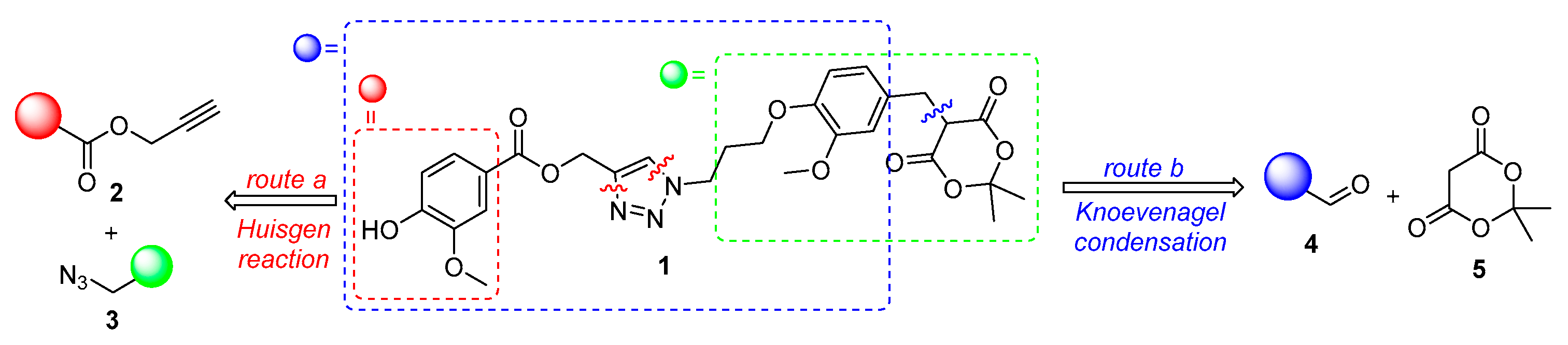
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis
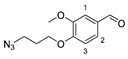
3.2.1. 4-(3-Azidopropoxy)-3-methoxybenzaldehyde (6)
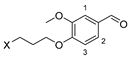
3.2.2. 4-(3-Halopropoxy)-3-methoxybenzaldehyde 9
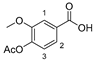
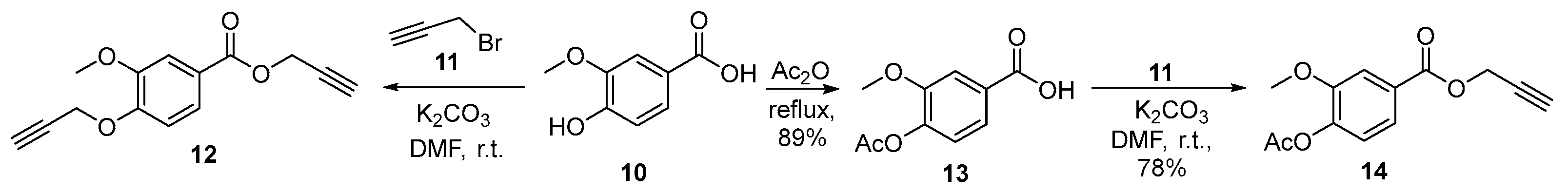
3.2.3. 4-Acetoxy-3-methoxybenzoic acid (13)
3.2.4. Prop-2-yn-1-yl 4-acetoxy-3-methoxybenzoate (14)
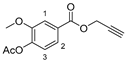
3.2.5. {1-[3-(4-Formyl-2-methoxyphenoxy)propyl]-1H-1,2,3-triazol-4-yl}methyl 4-acetoxy-3-methoxybenzoate (15)
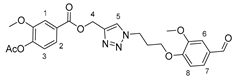
3.2.6. {1-[3-(4-Formyl-2-methoxyphenoxy)propyl]-1H-1,2,3-triazol-4-yl}methyl 4-hydroxy-3-methoxybenzoate (16)
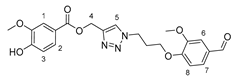
3.2.7. Arylidene Meldrum’s Acid Derivative 17
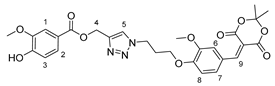
3.2.8. Arylmethyl Meldrum’s Acid Derivative 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mieriņa, I.; Peipiņa, E. Recent Applications of (Di)Alkyl Meldrum’s Acids (Microreview). Chem. Heterocycl. Compd. 2020, 56, 161–163. [Google Scholar] [CrossRef]
- Mieriņa, I.; Jure, M. Alkylidene and Arylidene Meldrum’s Acids as Versatile Reagents for the Synthesis of Heterocycles. Chem. Heterocycl. Compd. 2016, 52, 7–9. [Google Scholar] [CrossRef]
- Mierina, I. 5-Alkyl and 5,5-Dialkyl Meldrum’s Acids. Synlett 2014, 25, 155–156. [Google Scholar] [CrossRef][Green Version]
- Lotfi Nosood, Y.; Fattahi Ghahnavieh, N.; Takallou, A.; Rostami, A.; Ebrahimi, A.; Anwar, M.U.; Al-Harrasi, A. Microwave-Assisted [3 + 2] Cycloaddition Reactions of Dicyanoepoxides with Benzylidene Meldrum’s Acids. Org. Biomol. Chem. 2025, 23, 4084–4089. [Google Scholar] [CrossRef] [PubMed]
- Ryczkowska, M.; Trocka, A.; Hromova, A.; Makowiec, S. Meldrum’s Acid Assisted Formation of Tetrahydroquinolin-2-One Derivatives a Short Synthetic Pathway to the Biologically Useful Scaffold. Sci. Rep. 2024, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Fattahi Ghahnavieh, N.; Anwar, M.U.; Al-Harrasi, A.; Rostami, A. A One-Pot Two-Step Three-Component Stereoselective Mechanosynthesis of Ester-Functionalized Multisubstituted Δ2-Pyrazolines and Their Application in the Detection of Fluoride Ions. ACS Sustain. Chem. Eng. 2024, 12, 13750–13762. [Google Scholar] [CrossRef]
- Jiang, Z.; Kuninobu, Y. Synthesis of a Novel Twisted π-Conjugated Macrocycle via Double Friedel–Crafts Reaction and Its Physical Properties. Chem. Commun. 2024, 60, 7642–7645. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Ramis, X.; Salla, J.M.; Mantecón, A.; Serra, A. Anionic Copolymerization of Diglycidyl Ether of Bisphenol A with Meldrum’s Acid Derivatives Initiated by 4-(N,N-dimethylamino) Pyridine. J. Appl. Polym. Sci. 2009, 111, 1805–1815. [Google Scholar] [CrossRef]
- Huang, C.H.; Liu, Y.L. Self-Polymerization of Meldrum’s Acid-Amine Compounds: An Effective Route to Polyamides. Polym. Chem. 2021, 12, 291–298. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hu, C.C.; Chiu, Y.T.; Lai, J.Y.; Liu, Y.L. In Situ Crosslinking and Micro-Cavity Generation in Fabrication of Polymeric Membranes for Pervaporation Dehydration on Methanol Aqueous Solutions. J. Memb. Sci. 2018, 563, 371–379. [Google Scholar] [CrossRef]
- Heydari, A.; Hosseini, M.; Darroudi, M.; Behzadi, M.; Hronský, V.; Sučik, G.; Rouh, H.; Sheibani, H. Toward Efficient Functionalization of Polystyrene Backbone through Ketene Chemistry: Synthesis, Characterization, and DFT Study. Polym. Adv. Technol. 2023, 34, 587–596. [Google Scholar] [CrossRef]
- Yang, L.; Ruan, S.; Zhang, A.; Hu, M.; Zhang, J.; Sheng, K.; Tian, J.; Zhang, Y.; Wu, S.; Li, J. A Colorimetric and Ratiometric Fluorescent Probe with Meldrum’s Acid as the Recognition Group for in Vitro and in Vivo Imaging of Hypochlorite. Dye. Pigment. 2020, 175, 108144. [Google Scholar] [CrossRef]
- Li, H.; Wen, Z.; Jin, L.; Kan, Y.; Yin, B. A Coumarin–Meldrum’s Acid Conjugate Based Chemodosimetric Probe for Cyanide. Chem. Commun. 2012, 48, 11659–11661. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.L.; de Cordova, L.M.; Schramm, A.D.S.; Nicoleti, C.R.; Machado, V.G. Chromogenic and Fluorogenic Chemodosimeter Derived from Meldrum’s Acid Detects Cyanide and Sulfide in Aqueous Medium. J. Mol. Liq. 2019, 282, 142–153. [Google Scholar] [CrossRef]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Balaguer, P.; Allais, F. Innovative Bio-Based Organic UV-A and Blue Light Filters from Meldrum’s Acid. Molecules 2020, 25, 2178. [Google Scholar] [CrossRef]
- Wang, S.; Kim, S.H. New Solvatochromic Merocyanine Dyes Based on Barbituric Acid and Meldrum’s Acid. Dye. Pigment. 2009, 80, 314–320. [Google Scholar] [CrossRef]
- Khatoon, S.; Mehfooz, H.; Shehzadi, S.A.; Saeed, A.; Kalsoom, S.; Channar, P.A.; Ismail, H.; Rauf, A.; Naveed, S.; Taslimi, P.; et al. Synthesis, Anti-Enzymatic Appraisal, In Silico, and SAR Analysis of 5-Aryl/Heteroaryl-2,2-Dimethyl-1,3-Dioxane-4,6-Dione Derivatives. ChemistrySelect 2025, 10, e202404787. [Google Scholar] [CrossRef]
- Araújo, I.M.; Pereira, R.L.S.; de Araújo, A.C.J.; Gonçalves, S.A.; Tintino, S.R.; de Morais Oliveira-Tintino, C.D.; de Menezes, I.R.A.; Salamoni, R.; Begnini, I.M.; Rebelo, R.A.; et al. In Vitro and in Silico Effect of Meldrum’s Acid-Derived Compounds on Staphylococcus Aureus Strains as NorA Efflux Pump Inhibitors. Biophys. Chem. 2025, 316, 107344. [Google Scholar] [CrossRef]
- Vanga, M.K.; Bhukya, R.; Thumma, V.; Ambadipudi, S.S.S.S.S.; Nayak, V.L.; Andugulapati, S.B.; Manga, V. Design and Synthesis of Meldrum’s Acid Based 7-Azaindole Anchored 1,2,3-Triazole Hybrids as Anticancer Agents. RSC Med. Chem. 2024, 15, 1709–1721. [Google Scholar] [CrossRef]
- Mierina, I.; Jure, M.; Zeberga, S.; Makareviciene, V.; Zicane, D.; Tetere, Z.; Ravina, I. Novel Type of Carbon-centered Antioxidants Arylmethyl Meldrum’s Acids—Inhibit Free Radicals. Eur. J. Lipid Sci. Technol. 2017, 119, 1700172. [Google Scholar] [CrossRef]
- Mieriņa, I.; Peipiņa, E.; Aišpure, K.; Jure, M. 1st Generation Dendrimeric Antioxidants Containing Meldrum’s Acid Moieties as Surface Groups. New J. Chem. 2022, 46, 607–620. [Google Scholar] [CrossRef]
- Bērziņa, L.; Mieriņa, I. Antiradical and Antioxidant Activity of Compounds Containing 1,3-Dicarbonyl Moiety: An Overview. Molecules 2023, 28, 6203. [Google Scholar] [CrossRef] [PubMed]
- Bērziņa, L.; Mieriņa, I. Vanillic and Meldrum’s Acid Containing Antioxidant. Key Eng. Mater. 2022, 933, 162–168. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the Vanillin Synthesis and Biotransformation: A Review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering Multifaceted Role of Vanillic Acid beyond Flavours: Nutraceutical and Therapeutic Potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Xu, Z.; Li, J. An Efficient One-Pot Synthesis of Pyrano[3,2-c]Quinolin-2,5-Dione Derivatives Catalyzed by l-Proline. Molecules 2012, 17, 13856–13863. [Google Scholar] [CrossRef]
- List, B.; Castello, C. A Novel Proline-Catalyzed Three-Component Reaction of Ketones, Aldehydes, and Meldrum’s Acid. Synlett 2001, 2001, 1687–1689. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Abdelgawad, M.A.; Ahmed, N.; Amjad, M.W.; Hussain, M.A.; Elsherif, M.A.; Ejaz, H.; Alotaibi, N.H.; Filipović, I.; Janković, N. Synthesis, Characterization, and Biological Evaluation of Meldrum’s Acid Derivatives: Dual Activity and Molecular Docking Study. Pharmaceuticals 2023, 16, 281. [Google Scholar] [CrossRef]
- Attanasi, O.; FiIippone, P.; Mei, A. Effect of Metal Ions In Organic Synthesis. Part XVI. Knoevenagel Condensations of Aldehydes and Tosylhydrazones with 2,4-Pentanedi One by Copper (II) Chloride-Catalyzed Reaction. Synth. Commun. 1983, 13, 1203–1208. [Google Scholar] [CrossRef]
- Sysoeva, A.A.; Il’in, M.V.; Bolotin, D.S. Cooperative Covalent–Noncovalent Organocatalysis of the Knoevenagel Condensation Based on an Amine and Iodonium Salt Mixture. ChemCatChem 2024, 16, e202301668. [Google Scholar] [CrossRef]
- Saghian, M.; Dehghanpour, S.; Bayatani, Z. A Facile, Rapid Procedure for Knoevenagel Condensation Reaction Catalyzed by Efficient Amino-Bifunctional Frameworks under Mild Conditions. Sci. Rep. 2023, 13, 15563. [Google Scholar] [CrossRef]
- McNab, H. Meldrum’s Acid. Chem. Soc. Rev. 1978, 7, 345. [Google Scholar] [CrossRef]
- Brosge, F.; Singh, P.; Almqvist, F.; Bolm, C. Selected Applications of Meldrum’s Acid—A Tutorial. Org. Biomol. Chem. 2021, 19, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C. Meldrum’s Acid in Organic Synthesis. Heterocycles 1991, 32, 529–597. [Google Scholar] [CrossRef]
- Janković, N.; Bugarčić, Z.; Marković, S. Double Catalytic Effect of (PhNH3)2CuCl4 in a Novel, Highly Efficient Synthesis of 2-Oxo- and Thioxo-1,2,3,4-Tetrahydropyrimidines. J. Serbian Chem. Soc. 2015, 80, 595–604. [Google Scholar] [CrossRef]
- Kamal, A.; Prabhakar, S.; Janaki Ramaiah, M.; Venkat Reddy, P.; Ratna Reddy, C.; Mallareddy, A.; Shankaraiah, N.; Lakshmi Narayan Reddy, T.; Pushpavalli, S.N.C.V.L.; Pal-Bhadra, M. Synthesis and Anticancer Activity of Chalcone-Pyrrolobenzodiazepine Conjugates Linked via 1,2,3-Triazole Ring Side-Armed with Alkane Spacers. Eur. J. Med. Chem. 2011, 46, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Kyriukha, Y.A.; Afitska, K.; Kurochka, A.S.; Sachan, S.; Galkin, M.; Yushchenko, D.A.; Shvadchak, V.V. α-Synuclein Dimers as Potent Inhibitors of Fibrillization. J. Med. Chem. 2019, 62, 10342–10351. [Google Scholar] [CrossRef]
- Ryu, E.K.; Choe, Y.S.; Lee, K.-H.; Choi, Y.; Kim, B.-T. Curcumin and Dehydrozingerone Derivatives: Synthesis, Radiolabeling, and Evaluation for β-Amyloid Plaque Imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bērziņa, L.; Mieriņa, I. Synthesis of Vanillic Acid—Meldrum’s Acid Conjugate. Molbank 2025, 2025, M2005. https://doi.org/10.3390/M2005
Bērziņa L, Mieriņa I. Synthesis of Vanillic Acid—Meldrum’s Acid Conjugate. Molbank. 2025; 2025(2):M2005. https://doi.org/10.3390/M2005
Chicago/Turabian StyleBērziņa, Laima, and Inese Mieriņa. 2025. "Synthesis of Vanillic Acid—Meldrum’s Acid Conjugate" Molbank 2025, no. 2: M2005. https://doi.org/10.3390/M2005
APA StyleBērziņa, L., & Mieriņa, I. (2025). Synthesis of Vanillic Acid—Meldrum’s Acid Conjugate. Molbank, 2025(2), M2005. https://doi.org/10.3390/M2005