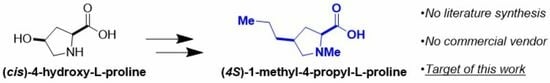
Stereoselective Preparation of (4S)-1-Methyl-4-propyl-L-proline Commencing from (cis)-4-Hydroxy-L-proline
Abstract
1. Introduction
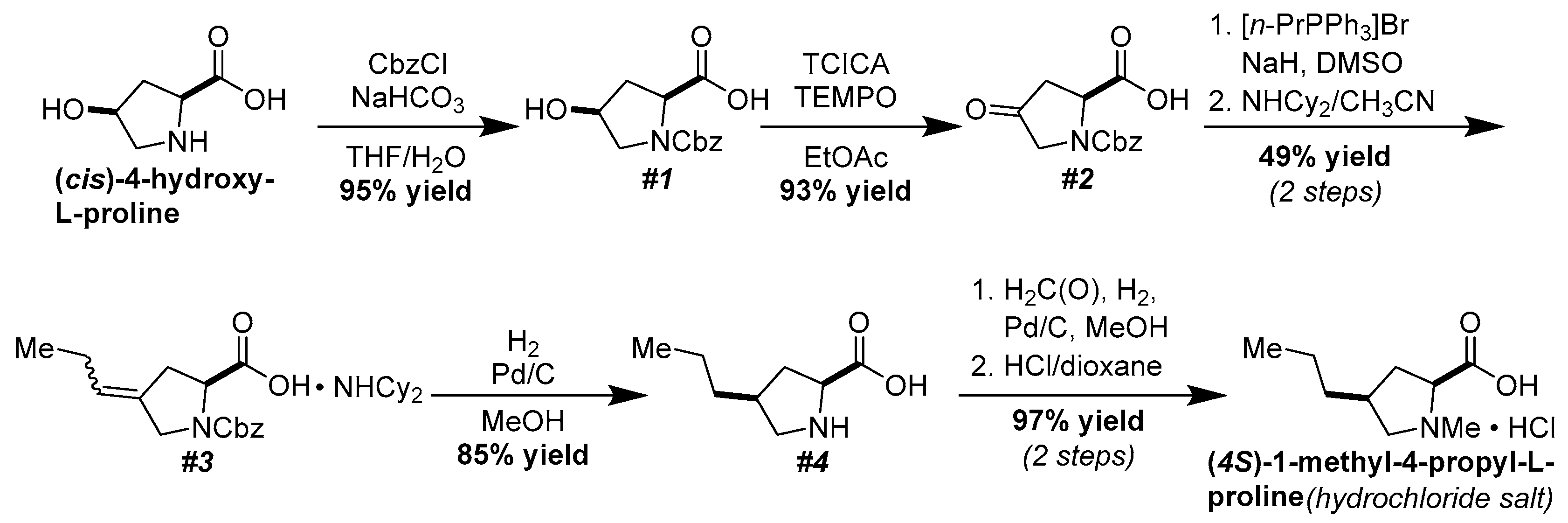
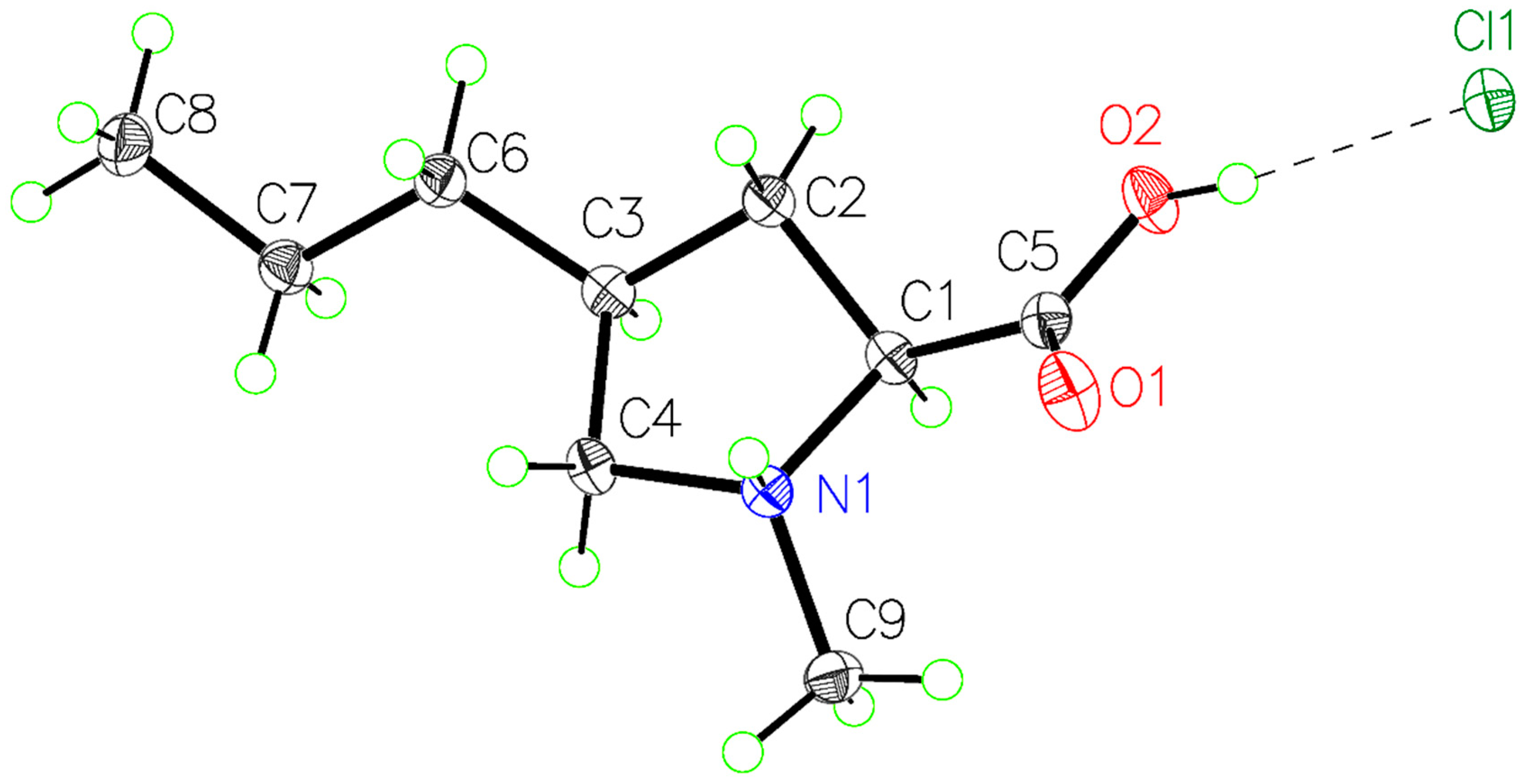
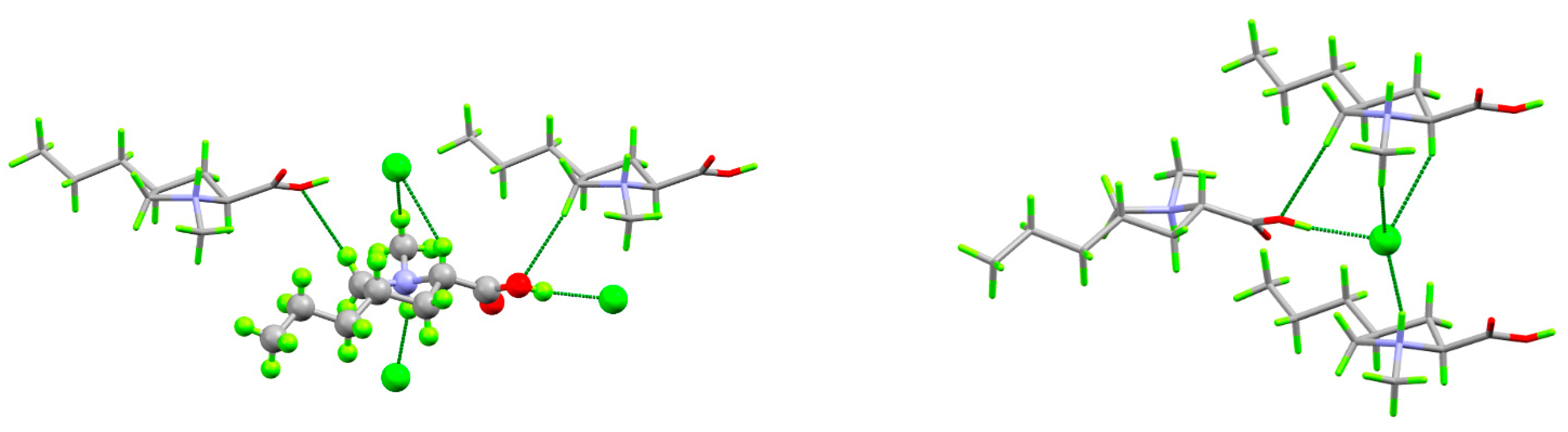
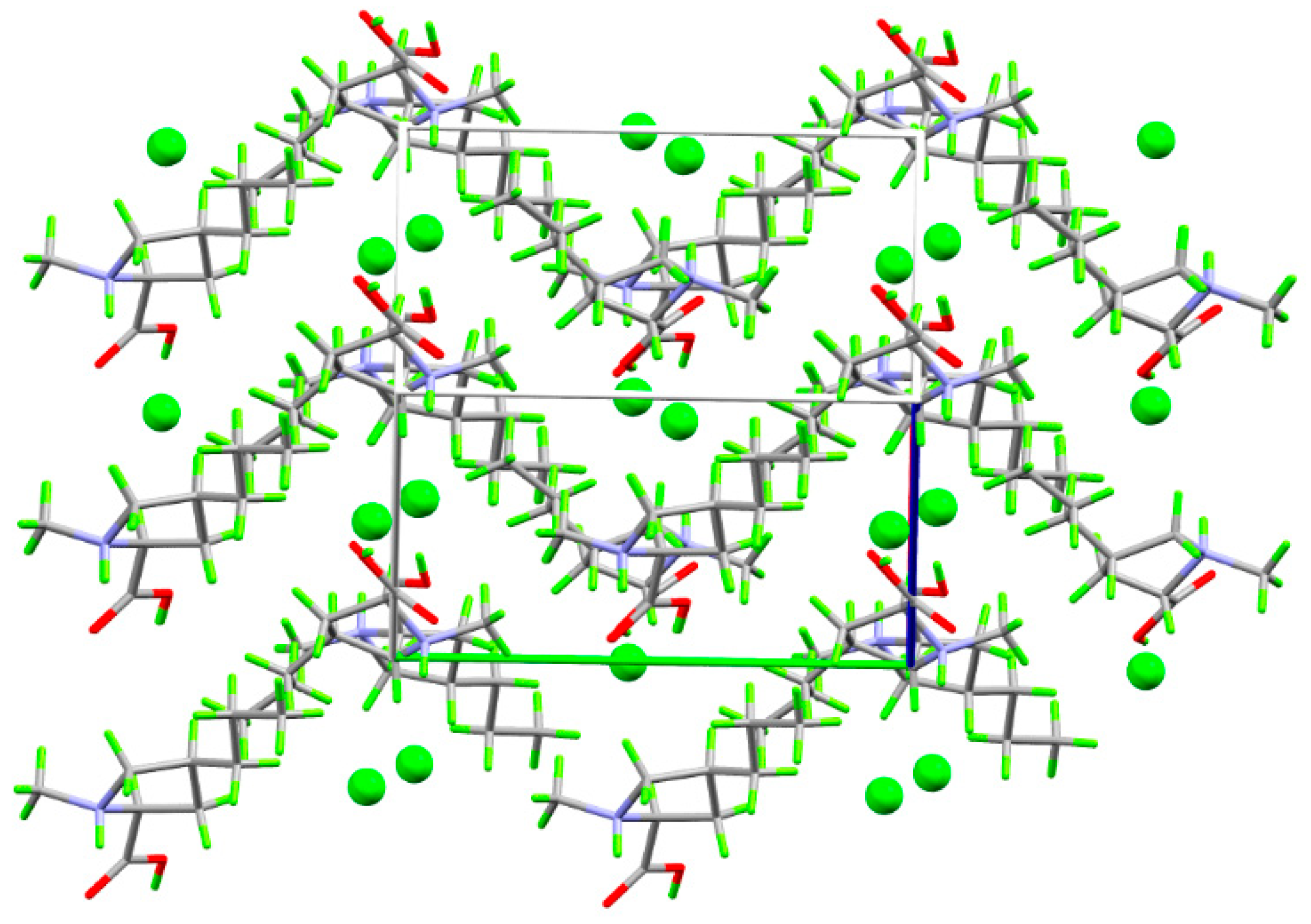
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef] [PubMed]
- Privalsky, T.M.; Soohoo, A.M.; Wang, J.; Walsh, C.T.; Wright, G.D.; Gordon, E.M.; Gray, N.S.; Khosla, C. Prospects for Antibacterial Discovery and Development. J. Am. Chem. Soc. 2021, 143, 21127–21142. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Boger, D.L. Maxamycins: Durable Antibiotics Derived by Rational Redesign of Vancomycin. Acc. Chem. Res. 2020, 53, 2587–2599. [Google Scholar] [CrossRef]
- Mason, J.D.; Terwilliger, D.W.; Pote, A.R.; Myers, A.G. Practical Gram-Scale Synthesis of Iboxamycin, a Potent Antibiotic Candidate. J. Am. Chem. Soc. 2021, 143, 11019–11025. [Google Scholar] [CrossRef]
- Mandhapati, A.R.; Yang, G.; Kato, T.; Shcherbakov, D.; Hobbie, S.N.; Vasella, A.; Böttger, E.C.; Crich, D. Structure-Based Design and Synthesis of Apramycin–Paromomycin Analogues: Importance of the Configuration at the 6′-Position and Differences between the 6′-Amino and Hydroxy Series. J. Am. Chem. Soc. 2017, 139, 14611–14619. [Google Scholar] [CrossRef]
- Sathyamoorthi, S. Fun with Unusual Functional Groups: Sulfamates, Phosphoramidates, and Di-tert-butyl Silanols. Eur. J. Org. Chem. 2024, 27, e202301283. [Google Scholar] [CrossRef]
- Joshi, H.; Nirpal, A.K.; Paul, D.; Kelley, S.P.; Mague, J.T.; Sathyamoorthi, S. The Development of a Sulfamate-Tethered Aza-Michael Cyclization Allows for the Preparation of (−)-Negamycin tert-Butyl Ester. J. Org. Chem. 2024, 89, 5911–5916. [Google Scholar] [CrossRef]
- Mandal, G.H.; Kelley, S.P.; Sathyamoorthi, S. Enantioselective Total Syntheses of (+)-Kasugamycin and (+)-Kasuganobiosamine Highlighting a Sulfamate-Tethered Aza-Wacker Cyclization Strategy. Org. Lett. 2024, 26, 5463–5466. [Google Scholar] [CrossRef]
- Mandal, G.H.; Sathyamoorthi, S. Sulfamate Tethered Aza-Wacker Strategy for a Kasugamine Synthon. J. Org. Chem. 2024, 89, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Abe, I. Lincosamide Antibiotics: Structure, Activity, and Biosynthesis. ChemBioChem 2024, 25, e202300840. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.J.; Ross, H.B.; Ozere, R.L.; Digout, G.; Van, R. Lincomycin: A New Antibiotic Active Against Staphylococci And Other Gram-Positive Cocci: Clinical And Laboratory Studies. Can. Med. Assoc. J. 1964, 91, 1056–1060. [Google Scholar]
- Pyörälä, S.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Greko, C.; Moreno, M.A.; Pomba, M.C.M.F.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance. Vet. J. 2014, 200, 230–239. [Google Scholar] [CrossRef]
- Collin, M.-P.; Hobbie, S.N.; Böttger, E.C.; Vasella, A. Synthesis of 1,2,3-Triazole Analogues of Lincomycin. Helv. Chim. Acta 2008, 91, 1838–1848. [Google Scholar] [CrossRef]
- Brahme, N.M.; Gonzalez, J.E.; Rolls, J.P.; Hessler, E.J.; Mizsak, S.; Hurley, L.H. Biosynthesis of the lincomycins. 1. Studies using stable isotopes on the biosynthesis of the propyl- and ethyl-L-hygric acid moieties of lincomycins A and B. J. Am. Chem. Soc. 1984, 106, 7873–7878. [Google Scholar] [CrossRef]
- Slomp, G.; MacKellar, F.A. Lincomycin. IV. Nuclear magnetic resonance studied on the structure of lincomycin, its degradation products, and some analogs. J. Am. Chem. Soc. 1967, 89, 2454–2459. [Google Scholar] [CrossRef]
- Magerlein, B.J.; Birkenmeyer, R.D.; Herr, R.R.; Kagan, F. Lincomycin. V. Amino acid fragment. J. Am. Chem. Soc. 1967, 89, 2459–2464. [Google Scholar] [CrossRef]
- Gandi, V.R.; Doan, B.N.D.; Kasinathan, S.; Bates, R.W. Stereocontrol in the synthesis of cyclic amino acids: A new ligand for directed hydrogenation through hydrogen bonding. Org. Biomol. Chem. 2019, 17, 2753–2758. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, D.; Wiener, J.; Tang, S.; Chepyshev, S.; Schafmeister, C. Development of Fmoc-Protected Bis-Amino Acids toward Automated Synthesis of Highly Functionalized Spiroligomers. Org. Lett. 2022, 24, 3421–3425. [Google Scholar] [CrossRef]
- Jones, G.P.; Naidu, B.P.; Paleg, L.G.; Tiekink, E.R.T. Hygric acid (I) and stachydrine (II) as their hydrochlorides. Acta Crystallogr. Sect. C 1988, 44, 1669–1671. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Newberry, R.W.; Raines, R.T. n→π* Interactions Engender Chirality in Carbonyl Groups. Org. Lett. 2014, 16, 3421–3423. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.F.; Howard, K.J.; Snaith, J.S.; Blake, A.J.; Li, W.-S.; Steel, P.J. An enantioselective route to pyrrolidines: Removal of the chiral template from homochiral pyrroloimidazoles. Tetrahedron 2011, 67, 8925–8936. [Google Scholar] [CrossRef]
- de C. T. Carrondo, M.A.A.F.; Duarte, M.T.L.S.; Gonçalves, M.L.S.S.; O’Brien, P.; Hursthouse, M.B. Molecular and crystal structure of cis-bis(L-hydroxyprolinato)-copper(II) tetrahydrate and trans-bis(D-allohydroxyprolinato)-copper(II)–water(2/5). J. Chem. Soc. Dalton Trans. 1990, 213–217. [Google Scholar] [CrossRef]
- Yin, D.; Wang, L.; Yuan, Z. Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5. Z. Kristallogr. NCS 2023, 238, 1055–1056. [Google Scholar] [CrossRef]
- Costantini, N.V.; Ganguly, H.K.; Martin, M.I.; Wenzell, N.A.; Yap, G.P.A.; Zondlo, N.J. The Distinct Conformational Landscapes of 4S-Substituted Prolines That Promote an endo Ring Pucker. Chem. Eur. J. 2019, 25, 11356–11364. [Google Scholar] [CrossRef]
- Pinalli, R.; Brancatelli, G.; Pedrini, A.; Menozzi, D.; Hernández, D.; Ballester, P.; Geremia, S.; Dalcanale, E. The Origin of Selectivity in the Complexation of N-Methyl Amino Acids by Tetraphosphonate Cavitands. J. Am. Chem. Soc. 2016, 138, 8569–8580. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Kotch, F.W.; Choudhary, A.; Guzei, I.A.; Raines, R.T. The Aberrance of the 4S Diastereomer of 4-Hydroxyproline. J. Am. Chem. Soc. 2010, 132, 10857–10865. [Google Scholar] [CrossRef][Green Version]
- Mycock, D.K.; Glossop, P.A.; Lewis, W.; Hayes, C.J. A formal synthesis of (+)-lactacystin from 4-hydroxyproline. Tetrahedron Lett. 2013, 54, 55–57. [Google Scholar] [CrossRef]
- Jones, G.P.; Naidu, B.P.; Paleg, L.G.; Tiekink, E.R.T. Structures of three N-methylated 4-hydroxyproline derivatives. Acta Crystallogr. Sect. C 1988, 44, 2208–2211. [Google Scholar] [CrossRef]
- Yuan, J.; Cai, Z.-Q.; Huang, C.-J.; Xu, W.-R. (2R,4R)-1-(tert-Butoxycarbonyl)-4-methoxypyrrolidine-2-carboxylic acid. Acta Crystallogr. Sect. E 2010, 66, o3258. [Google Scholar] [CrossRef]
- Tamaki, M.; Han, G.; Hruby, V.J. Synthesis of 4-cis-Phenyl-l-proline via Hydrogenolysis. J. Org. Chem. 2001, 66, 3593–3596. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, G.H.; Choudhary, S.; Kelley, S.P.; Sathyamoorthi, S. Stereoselective Preparation of (4S)-1-Methyl-4-propyl-L-proline Commencing from (cis)-4-Hydroxy-L-proline. Molbank 2025, 2025, M2003. https://doi.org/10.3390/M2003
Mandal GH, Choudhary S, Kelley SP, Sathyamoorthi S. Stereoselective Preparation of (4S)-1-Methyl-4-propyl-L-proline Commencing from (cis)-4-Hydroxy-L-proline. Molbank. 2025; 2025(2):M2003. https://doi.org/10.3390/M2003
Chicago/Turabian StyleMandal, Gour Hari, Shifali Choudhary, Steven P. Kelley, and Shyam Sathyamoorthi. 2025. "Stereoselective Preparation of (4S)-1-Methyl-4-propyl-L-proline Commencing from (cis)-4-Hydroxy-L-proline" Molbank 2025, no. 2: M2003. https://doi.org/10.3390/M2003
APA StyleMandal, G. H., Choudhary, S., Kelley, S. P., & Sathyamoorthi, S. (2025). Stereoselective Preparation of (4S)-1-Methyl-4-propyl-L-proline Commencing from (cis)-4-Hydroxy-L-proline. Molbank, 2025(2), M2003. https://doi.org/10.3390/M2003