Abstract
Herein we report the synthesis of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl 5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate. The compound was fully characterized by melting point, 1H-, 13C-NMR spectroscopy, IR spectroscopy, UV-VIS spectroscopy, mass spectrometry, and elemental analysis. The obtained data confirmed the successful synthesis and structure of the novel molecule.
1. Introduction
Riboflavin (Rf) has a critical role in the health and well-functioning of an organism [1] as the sole precursor of the co-enzymes that act as one or two electron carriers in a myriad of biological processes [2]. Being one of the B family of vitamins, Rf is metabolically activated to flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), critical in hundreds of redox, decarboxylation, and hydroxylation reactions, as well as electron transport, nitrogen homeostasis, and conversion of other vitamins to active forms and nitrogen cycles. Regular Rf intake through diet is necessary due to human inability to synthesize Rf and rapid turnover via excretion. Deficiency of Rf induces numerable metabolic disorders, embryonic and childhood developmental delays, and, in extreme cases, ariboflavinosis [3]. The importance of dietary riboflavin is underscored by the essential role of FMN or FAD in the activation of B6 and B9 vitamins and plasma homocysteine, as well as in synthesis of niacin from tryptophan [4].
Accurate detection of Rf is critical to many health-related fields such as human [5,6] and animal nutrition, food and drug quality, diagnosis and treatment of related ailments and diseases [7], pharmaceutical research [8], cancer diagnostics [9], and disease therapy [10,11]. One of the most common assays for riboflavin status and deficiency, the erythrocyte glutathione reductase activation coefficient (EGRAC) assay, uses an indirect methodology via an enzyme-coupled reaction in which blood FAD reduces glutathione [12]. Direct detection of Rf may be based on one of several characteristics: UV absorption peak at 450 nm due to isoalloxazine moiety, fluorescence absorption that is quenched if Rf is bound to the transport protein, and detectable electrochemical behavior due to the participation of 2e−/2H+ in the redox processes [13]. Among established methods [14], mass spectrometry (MS) has the sensitivity and selectivity to achieve the quantitative analysis of Rf biological samples such as blood with a very low content of vitamin [15,16,17,18]. However, interferences and co-elution peaks must be addressed by purification and separation methods [19] that require extensive resources and highly qualified personnel that are generally not readily accessible in populations susceptible of Rf deficiency. Few examples of indirect competitive ELISA immunoassays for Rf detection in food samples have been reported [20,21] with limited sensitivity. While these methods are useful for various applications, few are sufficiently sensitive or affordable, easy to use, or possess the stability to be employed for widespread detection or diagnosis.
It appears to us that there is a need for an affordable Rf detection method to be used in at-risk populations that do not have access to modern, sophisticated diagnostic health services, a balanced diet, or the benefit of a preventative health system. The key compound should simultaneously take advantage of the well-known biotin–streptavidin detection system and have tight binding to the chicken riboflavin transport protein (RBP) comparable to Rf. Based on binding studies reporting that biotin’s bicyclic ureido region and riboflavin’s isoalloxazine ring are vital for binding to the streptavidin and RBP, respectively, we coupled a Rf derivative void of OH groups and biotin through the side chains via an ester bond. We report here the synthesis of this compound, Scheme 1, which was characterized by NMR, IR, UV-VIS spectroscopy, mass spectrometry, and elemental analysis.
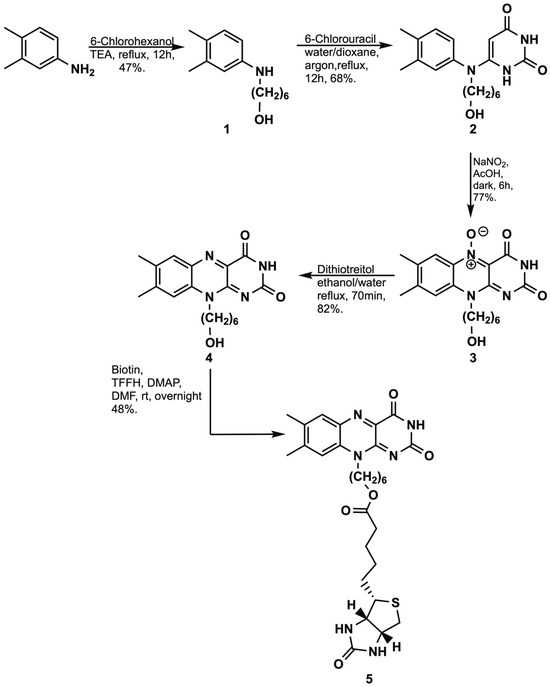
Scheme 1.
Reaction sequence and conditions for synthesis of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl 5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate.
2. Results
We synthesized the Rf derivative 4 following a modified published procedure [22]. A mixture of 3,4-dimethylaniline (3 eq.) and 6-chlorohexanol (1 eq.) in triethylamine were refluxed overnight. The crude product was purified by dry column chromatography with dichloromethane (CH2Cl2):methanol (98:2) eluent and recrystallized from hexanes to afford 1 in 47% yield. N-(6′-hydroxyhexyl)-3,4-dimethylaniline 1 (3 eq.) and 6-chlorouracil (1 eq) were refluxed under argon in water:dioxane (1:1) overnight. Following extraction, precipitation (pH = 3), and recrystallization from water, 2 was obtained in 68% yield. Cyclization of compound 2 (1 eq.) was performed in acetic acid by reaction overnight with NaNO2 (3 eq.) in the dark. Concentration to dryness followed by recrystallization from ethyl acetate–ethanol (1:1) afforded 3 in 77% yield. The isoalloxazine oxide derivative 3 was reduced with dithiothreitol in an ethanol/water solution with reflux. After solvent removal and recrystallization from ethanol, the riboflavin derivative 4 was obtained in 82% yield. Esterification of biotin with 4 was performed catalytically in DMF following a modified published protocol [23]. Extraction with CH2Cl2 followed by solvent removal and recrystallization from DMSO–water afforded the derivative 5 in 48% yield as a pale yellow-orange solid soluble in DMSO and DMF, insoluble in water or halogenated solvents such as chloroform or CH2Cl2, and very sparingly soluble in methanol.
The structure of 5 was verified by 1H and 13C NMR spectroscopy, mass spectrometry, UV-VIS spectroscopy, and elemental analysis. The discussed spectroscopic data are included in the supplemental material. The 1H NMR spectrum of 5 displayed the N-H peaks from the ureido region at 6.35 and 6.43 ppm, and the flavin’s NH at 11.30 ppm. On the tetrahydrothiophene ring, the CH2 protons α to the S were identified as a doublet at 2.58 and a doublet of doublets at 2.80 ppm, consistent with the corresponding resonances in the 1H NMR spectrum of free biotin. At the same time, the CH α to the S was observed at 3.08 ppm as a multiplet. The two CH protons neighboring the NH groups in the ureido bicycle appeared at 4.13 and 4.29 ppm, flanked by the triplets of the CH2-N and CH2O groups belonging to the riboflavin derivative tail. On the flavin moiety, the aromatic hydrogens and one methyl’s hydrogens were detected at 7.79 and 7.91 ppm, as well as 2.41 ppm, respectively; the other methyl’s hydrogens overlapped with the residual peak of the solvent, DMSO. The resonances observed in the 13C NMR spectrum were assigned tentatively based on previous experimental data on flavins. The C=O ester’s signal at 173.4 ppm, together with the three amidic resonances at 156.1, 160.4, and 163.2 ppm, as well as the imide ones at 147.1 and 150.4 ppm, support the synthesis of the target compound 5. The aromatic carbon atoms were in the range 131.11–137.50 ppm, with only the resonance corresponding to carbon ortho to the methyl group at 116.54 ppm. The tetrahydrothiophene ring exhibited peaks at 55.9, 59.0, and 61.0 ppm, while the CH2O from the ester group was present at 64.09 ppm. The remaining aliphatic carbons were in the range 21.0–33.8 ppm. The CH2-N riboflavin derivative tail and the CH-S from the tetrahydrothiophene ring were obscured by the residual solvent peak. The presence of the methyl groups was indicated by peaks at 19.0 and 19.5 ppm. The C=O bonds were present in the IR spectrum of 5 around 1688 cm−1 while the stretching vibrations corresponding to the N-H bonds showed at 3293 cm−1. The mass spectrum contained, in addition to the base peak at 569 (M + 1), two major peaks, m/z = 243 and 343, corresponding to biotin and 4 fragments, respectively, resulting from the cleavage of the ester bond. The UV spectrum of solution of 0.074 mM 5 in DMSO exhibited the crucial peaks at 273 nm (ε = 28,500), 344 nm (ε = 7200), and 448 nm (ε = 12,000) that are characteristic of the flavin moiety, which were also observed in the control UV spectrum of Rf (0.065 mM solution in DMSO) 273 nm (ε = 24,300), 347 nm (ε = 5600), and 449 nm (ε = 8700).
3. Materials and Methods
3.1. Chemistry
Reagents, materials, and solvents were purchased from MilliporeSigma, St. Louis, MO, USA, and used without further purification. Riboflavin derivative, N-(6′-hydroxyhexyl)isoalloxazine, was synthesized based on a published procedure [22] while the title compound, 5, was synthesized following a modified published protocol [23]. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer using the solvent peak as an internal reference, with chemical shifts expressed in ppm. The HRMS and fragmentation pattern was collected on a Waters Xevo G2-XS QTof mass spectrometer, Waters, Millford, MA, USA, with a flow injection method at 0.2 mL/min 95% methanol/5% water, EI method in ion positive mode. Melting points were determined using a MelTemp apparatus. The UV-VIS data was collected on a Shimadzu UV 2600i, Shimadzu, Columbia, MD, USA, in the range 250–600 nm with a 1 cm quartz cell and a 1 mm wide slit on a solution of 0.074 mM of 5 in DMSO. The IR spectrum was acquired on an AGILENT CARY 630 FTIR spectrometer, Agilent, Santa Clara, CA, USA.
3.1.1. Synthesis of N-(6′-Hydroxyhexyl)-3,4-dimethylaniline (1)
3,4-dimethylaniline (9.3 g, 76 mmol) and 6-chlorohexanol (3.4 g, 25 mmol) in triethylamine (15 mL) were stirred at reflux overnight. CH2Cl2 (200 mL) was added to the cold reaction mixture and the solution was stirred with NaHCO3 (10%, 40.0 mL). Following extraction with CH2Cl2 (2 × 100 mL), the combined organic layers were dried over MgSO4 and concentrated to dryness. The crude product was purified on a flash dry column with CH2Cl2: methanol (98:2) eluent and recrystallized from hexanes to afford 1 as a light brown solid (2.6 g, 11.6 mmol, 47% yield): m.p. 45.9–47.4 °C; 1H NMR (400 MHz, CDCl3) δ 6.95 (d, 1H, J = 8 Hz), 6.47 (d, 1H, J = 6.4 Hz), 6.42 (dd,1H, J = 6, 16 Hz), 3.68 (t,2H, J = 8 Hz), 3.12 (t, 2H, J = 8 Hz), 2.22 (s, 3H), 2.18 (s, 3H), 1.63 (m, 4H), 1.46 (m, 4H).
3.1.2. Synthesis of 6-[N-(6′-Hydroxyhexyl)-3,4-xylidino]uracil (2)
N-(6′-Hydroxyhexyl)-3,4-dimethylaniline 1 (2.2 g, 9.9 mmol) was added to water:dioxane (1:1, 22 mL) and refluxed under argon for 20 min. Then, 6-chlorouracil (0.5 g, 3.4 mmol) was added and the reflux continued overnight. The pH of the cold reaction mixture was adjusted to 11 with NaOH (10%) and the solution was extracted with CH2Cl2 (2 × 50 mL). Diluted HCl was added to the aqueous layer until the pH = 3 and the target compound precipitated. Recrystallization from water afforded a white solid (0.77 g, 2.4 mmol, 68%): m.p. 204.8–206.8 °C; 1H NMR (400 MHz, DMSO) δ 10.32 (br s,1H), 10.08 (br s, 1H), 7.23 (d, 1H, J = 8.0 Hz), 7.04 (s, 1H), 6.97 (dd, 1H, J = 7.9, 2.3 Hz), 4.33 (t, 1H, J = 5.2 Hz), 4.09 (s, 1H), 3.57 (t, 2H, J = 8 Hz), 3.36 (t, 2H, J = 8 Hz), 2.24 (s, 6H), 1.45–1.37 (m, 4H), 1.24 (m, 4 H).
3.1.3. Synthesis of Isoalloxazine 5-Oxide (3)
6-[N-(6′-Hydroxyhexyl)-3,4-xylidino]uracil 2 (0.8 g, 2.4 mmol) was stirred in acetic acid (6.3 g, 105 mmol, 6 mL). NaNO2 (0.8 g, 11.5 mmol) was added in the dark at room temperature. After 3 h, H2O (4.0 mL) was added and the reaction continued overnight in the dark. The reaction mixture was concentrated to dryness, the solid residue washed with water twice and recrystallized from ethyl acetate: ethanol (50:50) to afford 3 as a yellow solid (0.66 g, 1.8 mmol, 77%): m.p. 221–224 °C; 1H NMR (400 MHz, DMSO) δ 11.03 (s, 1H), 8.09 (s, 1H), 7.79 (s, 1H), 4.52 (t, 2H, J = 4 Hz), 4.38 (t, 1H, J = 4 Hz), 3.41 (m, 2H), 2.50 (s, 3H), 2.39 (s, 3H), 1.69 (m, 2H), 1.47–1.35 (m, 6H).
3.1.4. Synthesis of N-(6′-Hydroxyhexyl)isoalloxazine (4)
Isoalloxazine 5-Oxide 3 (0.56 g, 1.64 mmol) was suspended in ethanol (400 mL) and an aqueous solution of dithiothreitol (1.2 g, 7.4 mmol, 16 mL) was added with stirring and refluxed for 70 min, after which the solvents were removed in vacuo. The resulting residue was recrystallized from ethanol to afford 4 as an orange solid (0.44 g, 1.3 mmol, 82%): m.p. 272–274 °C; 1H NMR (400 MHz, DMSO) δ 11.29 (s, 1H), 7.91 (s, 1H), 7.79 (s, 1H), 4.57 (t, 2H, J = 4 Hz), 4.37 (t, 1H, J = 4 Hz), 3.40 (q, 2H, J = 8 Hz), 2.52 (s, 3H), 2.41 (s, 3H), 1.72 (m, 2H), 1.48–1.37 (m, 6H).
3.1.5. Synthesis of 6-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl 5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate (5)
To the ice-cold suspension of biotin (0.35 g, 1.42 mmol) and tetramethylfluoroformamidinium hexafluorophosphate (TFFH) (0.35 g, 1.33 mmol) in DMF (12 mL), triethylamine (1.38 g, 13.6 mmol) was added. The reaction mixture was stirred at room temperature for 1 h and N-(6′-hydroxyhexyl)isoalloxazine (0.44 g, 1.28 mmol) and DMAP (0.03 g, 0.29 mmol) were added, after which the stirring was continued overnight. The resulting orange suspension was diluted with DMF (10 mL) and filtered. The filtrate was diluted with water (25 mL) and extracted with CH2Cl2 (50 mL × 3). The combined organic layers were dried and the solvents removed in vacuo. The solid residue was recrystallized from DMSO–water to yield a yellow-orange solid (0.35 g, 0.62 mmol, 48%): m.p. 237–238 °C with decomposition; 1H NMR (400 MHz, DMSO) δ 11.30 (s, 1H), 7.91 (s, 1H), 7.79 (s, 1H), 6.42 (s, 1H), 6.35 (s, 1H), 4.58 (t, 2H, J = 12Hz), 4.29 (m, 1H), 4.13 (m, 1H), 4.02 (t, 2H, J = 4Hz), 3.08 (m, 1H), 2.79 (dd, 1H, J = 8, 12Hz), 2.58 (d, 1H, J = 12 Hz), 2.41 (s, 3H), 2.29 (t, 2H, J = 8Hz), 1.73 (m, 2H), 1.33–1.58 (m, 12H); 13C NMR (400 MHz, DMSO) δ 173.4 (OCOCH2), 163.2 (NHCONH), 160.4 (NCONH), 156.1 (CCONH), 150.4 (NCCO), 147.1 (NCN), 137.5, 136.2, 134.2, 131.2, 131.1 (aromatic C), 116.5 (CH3CCCN), 64.1 (CH2CH2O), 61.0 (NHCHCHS), 59.0 (NHCHCH2S), 55.9 (NHCHCHS), 33.8- 21.0 (N-CH2(CH2)4CH2O, CO(CH2)4CH), 19.5 (CH3), 19.1 (CH3); MS (EI+) m/z 569, 343, 243; IR (cm−1) 3298, 2927, 2801, 1690, 1579, 1547; Elem. analysis (%) C 58.89, H6.29, N 14.89, 0 14.35, S 5.42 (theor. C 59.14, H 6.38, N14.78, O 14.07, S 5.64); UV-VIS (DMSO) λmax = 273 nm (ε = 28,500), 344 nm (ε = 7200), 448 nm (ε = 12,000).
4. Conclusions
The synthesis of a riboflavin derivative coupled to a biotin with potential use for riboflavin detection was presented. The structure of the compound was confirmed through NMR, IR, and UV-VIS spectroscopies, mass spectrometry, and elemental analysis.
Supplementary Materials
Figure S1: 1H NMR spectrum 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5, (400 MHz, DMSO); Figure S2. 1H NMR spectrum 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridine 10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5, region 1–5 ppm (400 MHz, DMSO); Figure S3. 13C NMR spectrum 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5, (400MHz, DMSO); Figure S4. UV-VIS Spectrum of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5, (0.074 mM in DMSO); Figure S5. IR Spectrum of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5; Figure S6. High Resolution Mass Spectrum of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoate 5; Figure S7. Mass Spectrum Fragmentation Pattern of 6-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)hexyl-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno [3,4-d]imidazol-4-yl)pentanoate 5.
Author Contributions
Conceptualization, S.M. and M.B.; methodology, S.M. and M.B.; formal analysis, S.M. and M.B.; investigation, D.N., R.S. and T.B.; writing—original draft preparation, S.M. and M.B.; writing—review and editing, S.M. and M.B.; visualization, S.M.; supervision, S.M. and M.B.; project administration, S.M.; funding acquisition, S.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The spectroscopic data presented are available as Supplementary Material.
Acknowledgments
We acknowledge financial support from the University of Michigan-Dearborn Office of Sponsored Research (ORSP).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Rf | Riboflavin |
| MS | Mass Spectrometry |
| RBP | Riboflavin-Binding Protein |
| DMSO | Dimethyl Sulfoxide |
| TFFH | Tetramethylfluoroformamidinium Hexafluorophosphate |
| DMF | Dimethyl Formamide |
| DMAP | 4-Dimethylaminopyridine |
References
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Krčmová, L.M.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.J.; Gomes, C.M. Chapter 12—Riboflavin (vitamin B2) and mitochondrial energy. In Molecular Nutrition; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 225–244. [Google Scholar]
- McAuley, E.; McNulty, H.; Hughes, C.; Strain, J.J.; Ward, M. Riboflavin status, MTHFR genotype and blood pressure: Current evidence and implications for personalized nutrition. Proc. Nutr. Soc. 2016, 75, 405–414. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Henonen, M.; Hirsch, E.K.I.; Magelsdorf, I.; McArdle, H.J.; Naska, A.; et al. EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies 2017. Scientific Opinion on Dietary Reference Values for riboflavin. EFSA J. 2017, 15, 4919–4984. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Christodoulou, J.; Rahman, S. Disorders of riboflavin metabolism. J. Inherit. Metab. Dis. 2019, 42, 608–619. [Google Scholar] [CrossRef]
- Jaroensuk, J.; Chuaboon, L.; Kesornpun, C.; Chaiyen, P. Enzymes in riboflavin biosynthesis: Potential antibiotic drug targets. Arch. Biochem. Biophys. 2023, 748, 109762. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, X.; Chen, Y.; Zeng, X.; Liu, J.; Zhao, Z.; Yang, H.; Zhang, Q.; Li, J.; Guo, Z.; et al. Development and Evaluation of [68Ga]Ga-Labeled Riboflavin Derivative for RFVT3-Targeted PET Imaging of Melanoma in Mice. Mol. Pharm. 2024, 21, 4960–4969. [Google Scholar] [CrossRef]
- Nisco, A.; Tolomeo, M.; Scalise, M.; Zanier, K.; Barile, M. Exploring the impact of flavin homeostasis on cancer cell metabolism. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189149. [Google Scholar] [CrossRef]
- Tutino, V.; Defrancesco, M.L.; Tolomeo, M.; De Nunzio, V.; Lorusso, D.; Paleni, D.; Caruso, M.G.; Notarnicola, M.; Barile, M. The Expression of Riboflavin Transporters in Human Colorectal Cancer. Anticancer Res. 2018, 38, 2659–2667. [Google Scholar] [CrossRef]
- Parkington, D.A.; Koulman, A.; Jones, K.S. Protocol for measuring erythrocyte glutathione reductase activity coefficient to assess riboflavin status. STAR Protoc. 2023, 4, 102726. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.M.; van Dijk, M.; Goorden, S.M.I. Riboflavin (B2) and FAD/FMN Metabolites. In Laboratory Guide to the Methods in Biochemical Genetics; Blau, N., Vaz, F.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Zhou, T.; Li, H.; Shang, M.; Sun, D.; Liu, C.; Che, G. Recent analytical methodologies and analytical trends for riboflavin (vitamin B2) analysis in food, biological and pharmaceutical samples. Trends Anal. Chem. 2021, 143, 116412. [Google Scholar] [CrossRef]
- Kaede Sasaki, K.; Hideo Hatate, H.; Ryusuke Tanaka, R. Determination of 13 Vitamin B and the related compounds using HPLC with UV detection and application to food supplements. Chromatographia 2020, 83, 839–851. [Google Scholar] [CrossRef]
- Akca, S.A.; Sargun, H.S.; Mizrak, O.F.; Yaman, M. Determination and assessment of the bioaccessibility of vitamins B1, B2, and B3 in commercially available baby foods. Microchem. J. 2019, 150, 104192. [Google Scholar] [CrossRef]
- Cellar, N.A.; McClure, S.C.; Salvati, L.M.; Reddy, T.M. A new sample preparation and separation combination for precise, accurate, rapid, and simultaneous determination of vitamins B1, B2, B3, B5, B6, B7, and B9 in infant formula and related nutritionals by LC-MS/MS. Anal. Chim. Acta 2016, 9334, 180–185. [Google Scholar] [CrossRef]
- McClure, S. Simultaneous Determination of Total Vitamins B1, B2, B3, and B6 in Infant Formula and Related Nutritionals by Enzymatic Digestion and LC-MS/MS-A Multi-Laboratory Testing Study Final Action: AOAC Method 2015.14. J. AOAC Int. 2020, 103, 1060–1072. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, H.L.; Yin, X.L.; Gu, H.W.; Xiao, R.; Xie, L.X.; Liu, Z.; Fang, H.; Wang, L.; Yu, R.Q. Rapid and interference-free analysis of nine B-group vitamins in energy drinks using trilinear component modeling of liquid chromatography-mass spectrometry data. Talanta 2018, 180, 108–119. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, S.; Lai, X.; Peng, J.; Lai, W. Developmental trend of immunoassays for monitoring hazards in food samples: A review. Trends Food Sci. Technol. 2021, 111, 68–88. [Google Scholar] [CrossRef]
- Yang, H.; Xu, W.; Liang, X.; Yang, Y.; Zhou, Y. Carbon nanotubes in electrochemical, colorimetric, and fluorometric immunosensors and immunoassays: A review. Microchim. Acta 2020, 187, 206. [Google Scholar] [CrossRef]
- Frier, C.; Dècout, J.L. Nucleotides and Flavin Method for Preparing New Flavin Derivatives: Synthesis of Flavin-Thymine -Oligonucleotide Adducts. J. Org. Chem 1997, 62, 3520–3528. [Google Scholar] [CrossRef]
- Pittelkow, M.; Kamounah, F.S.; Boas, U.; Pederson, B.; Christensen, J.B. TFFH as an Excellent Reagent for Acylation of Alcohols, Thiols and Dithiocarbamates. Synthesis 2004, 15, 2485–2492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).