(1S,1′S)-2,2′-(Benzylazanediyl)bis(1-((3aR,4R,6aR)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)ethan-1-ol)
Abstract
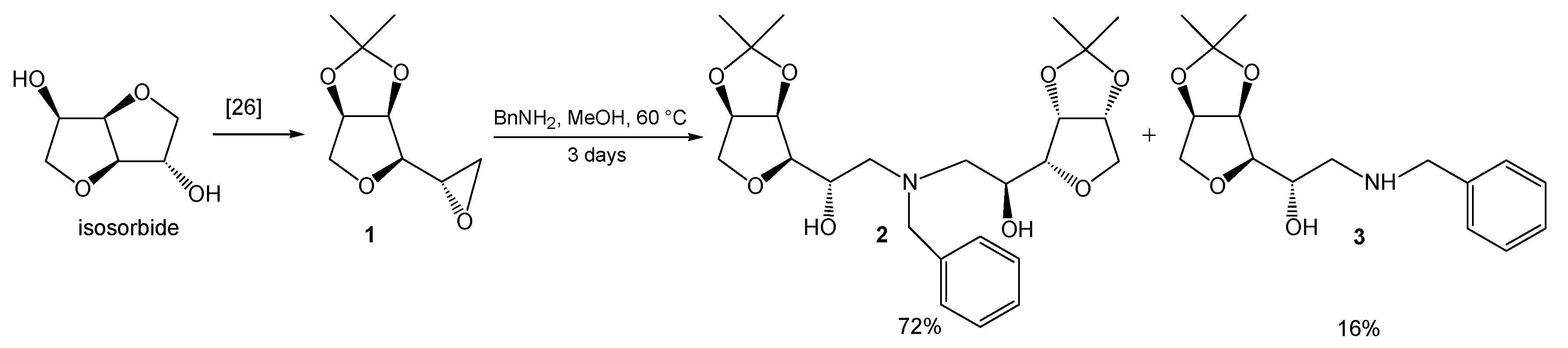
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yar, M.; Mushtaq, N.; Afzal, S. Synthesis, reactions, applications, and biological activity of diethanolamine and its derivatives. Russ. J. Org. Chem. 2013, 49, 949–967. [Google Scholar] [CrossRef]
- Toom, L.; Villo, P.; Liblikas, I.; Vares, L. Synthesis of Amphiphilic Amino Alcohols. Synth. Commun. 2008, 38, 4295–4313. [Google Scholar] [CrossRef]
- Jen, A.K.-Y.; Drost, K.J.; Cai, Y.; Rao, V.P.; Dalton, L.R. Thermally stable non linear optical polyimides: Synthesis and electro-optic properties. J. Chem. Soc. Chem. Commun. 1994, 965–966. [Google Scholar] [CrossRef]
- Sundararajan, G.; Narayanasamy, P. A New Polymer-Anchored Chiral Catalyst for Asymmetric Michael Addition Reactions. Org. Lett. 2001, 3, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Boogers, J.A.F.; Klaase, P.T.A.; de Vlieger, J.J.; Tinnemans, A.H.A. Crosslinked polymer materials for nonlinear optics. 2. Polyurethanes bearing azobenzene dyes. Macromolecules 1994, 27, 205–209. [Google Scholar] [CrossRef]
- Tuerel, T.; Tomovic, Z. Chemically Recyclable and Upcyclable Epoxy Resins Derived from Vanillin. ACS Sustain. Chem. Eng. 2023, 11, 8308–8316. [Google Scholar] [CrossRef]
- Cribiu, R.; Allevi, P.; Anastasia, M. Reduced collagen cross links: The first synthesis of all the possible (2S,2′S)-stereoisomers of 5-hydroxylysinonorleucine and of 5,5′-dihydroxylysinonorleucine in enantiomerically pure form. Tetrahedron Asymmetry 2005, 16, 3059–3069. [Google Scholar] [CrossRef]
- Khandavalli, P.C.; Spiess, O.; Boehm, O.M.; Freifeld, I.; Kesseler, K.; Jas, G.; Schinzer, D. Synthesis of Desfluorinated Nebivolol Isomers. J. Org. Chem. 2015, 80, 3965–3973. [Google Scholar] [CrossRef]
- Piskun, Y.A.; Vasilenko, I.V.; Kostjuk, S.V.; Zaitsev, K.V.; Zaitseva, G.S.; Karlov, S.S. Titanium Complexes of Dialkanolamine Ligands as Initiators for Living Ring-Opening Polymerization of e-Caprolactone. J. Polym. Sci. A Polym. Chem. 2010, 48, 1230–1240. [Google Scholar] [CrossRef]
- Dakshinamoorthy, D.; Peruch, F. Block and random copolymerization of ε-caprolactone, L-, and rac-lactide using titanium complex derived from aminodiol ligand. J. Polym. Sci. A Polym. Chem. 2012, 50, 2161–2171. [Google Scholar] [CrossRef]
- Dakshinamoorthy, D.; Peruch, F. Titanium complexes based on aminodiol ligands for the ring-opening polymerization of ε-caprolactone, rac-β-butyrolactone, and trimethylene carbonate. J. Polym. Sci. A Polym. Chem. 2011, 49, 5176–5185. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.; Hwang, S.-H.; Kim, D.; Hong, S.H.; Choi, T.-L. Modulating the Rate of Controlled Suzuki-Miyaura Catalyst-Transfer Polymerization by Boronate Tuning. Macromolecules 2022, 55, 3476–3483. [Google Scholar] [CrossRef]
- Deivasagayam, D.; Peruch, F. Titanium complexes based on aminodiol ligands for the ring opening polymerization of l- and d,l-lactide. Polymer 2011, 52, 4686–4693. [Google Scholar] [CrossRef]
- Manivannan, R.; Sundararajan, G. Latent Bimodal Polymerization of 1-Hexene by a Titanium-Based Diastereomeric Catalyst Containing a rac/meso-Aminodiol Ligand. Macromolecules 2002, 35, 7883–7890. [Google Scholar] [CrossRef]
- Zhang, A.-L.; Yang, L.-W.; Yang, N.-F.; Liu, Y.-L. The synthesis of chiral amino diol tridentate ligands and their enantioselective induction during the addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2014, 25, 289–297. [Google Scholar] [CrossRef]
- Tangellamudi, N.D.; Govindarajan, S. Unexpected chemoselectivity of cyclic enones on introducing additional chirality in diethanolamine ligand in catalytic asymmetric Michael addition reactions using heterobimetallics. J. Mol. Catal. A Chem. 2009, 314, 71–80. [Google Scholar] [CrossRef]
- Wu, P.; Celik, C.; Santoni, G.; Dallery, J.; Rehder, D. Sulfoxygenation catalysed by oxidovanadium complexes. Eur. J. Inorg. Chem. 2008, 33, 5203–5213. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Wang, H.-C.; Wang, Y.; Zheng, C.; You, S.-L. Enantioselective Dearomatization of Indoles via SmI-Mediated Intermolecular Reductive Coupling with Ketones. J. Am. Chem. Soc. 2023, 145, 10314–10321. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Bermeshev, M.V.; Samsonov, A.A.; Oprunenko, J.F.; Churakov, A.V.; Howard, J.A.L.; Karlov, S.S.; Zaitseva, G.S. Titanium complexes based on chiral enantiopure dialkanolamines: Synthesis, structures and catalytic activity. New J. Chem. 2008, 32, 1415–1431. [Google Scholar] [CrossRef]
- Meric, N.; Kayan, C.; Gurbuz, N.; Karakaplan, M.; Binbay, N.E.; Aydemir, M. New functional chiral P-based ligands and application in ruthenium-catalyzed enantioselective transfer hydrogenation of ketones. Tetrahedron Asymmetry 2017, 28, 1739–1749. [Google Scholar] [CrossRef]
- Prabagaran, N.; Abraham, S.; Sundararajan, G. Asymmetric Michael addition reaction using a chiral catalyst containing amino diol. ARKIVOC 2002, 7, 212–226. [Google Scholar] [CrossRef]
- Abraham, S.; Sundararajan, G. Investigation of the active species in a Michael addition promoted by chirally modified tetrahydroborate. Tetrahedron 2006, 62, 1474–1478. [Google Scholar] [CrossRef]
- Palchykov, V.A.; Gaponova, R.G.; Omelchenko, I.V.; Kasyan, L.I. Synthesis of new azapolycyclic scaffolds via the domino aminolysis of dicyclopentadiene diepoxide in water. Tetrahedron Lett. 2020, 61, 152097. [Google Scholar] [CrossRef]
- Pal’chikov, V.A.; Mykolenko, S.Y.; Pugach, A.N.; Zubkov, F.I. Composition and reactivity of aminolysis products of phenyl glycidyl ether with benzylamine. Russ. J. Org. Chem. 2017, 53, 656–662. [Google Scholar] [CrossRef]
- Randall, K.A.; Frazier, J.; Moore, L.L.; Weigel, L.O. Alkylation of N-trimethylsilylated primary amines with arylethylene oxides. An efficient synthesis of 1-phenethanolamines. Tetrahedron Lett. 1986, 27, 2451–2454. [Google Scholar] [CrossRef]
- Boiaryna, L.; Guillarme, S.; Saluzzo, C. 5(S)-((3aR,4R,6aR)-2,2-Dimethyltetrahydrofuro [3,4-d][1,3]dioxol-4-yl)-2-phenyl-4,5-dihydrooxazole]. Molbank 2024, 2024, M1843. [Google Scholar] [CrossRef]
- Guillarme, S.; Nguyen, T.X.M.; Saluzzo, C. New chiral ligands from isosorbide: Application in asymmetric transfer hydrogenation. Tetrahedron Asymmetry 2008, 19, 1450–1454. [Google Scholar] [CrossRef]
- Le, T.T.; Guillarme, S.; Saluzzo, C. New class of beta aminoalcohol ligands derived from isosorbide and isomannide: Application in hydrogen transfer reduction of prochiral ketones. Tetrahedron 2010, 66, 8893–8898. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadraoui, M.; Guillarme, S.; Saluzzo, C. (1S,1′S)-2,2′-(Benzylazanediyl)bis(1-((3aR,4R,6aR)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)ethan-1-ol). Molbank 2025, 2025, M1962. https://doi.org/10.3390/M1962
Kadraoui M, Guillarme S, Saluzzo C. (1S,1′S)-2,2′-(Benzylazanediyl)bis(1-((3aR,4R,6aR)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)ethan-1-ol). Molbank. 2025; 2025(1):M1962. https://doi.org/10.3390/M1962
Chicago/Turabian StyleKadraoui, Mohammed, Stéphane Guillarme, and Christine Saluzzo. 2025. "(1S,1′S)-2,2′-(Benzylazanediyl)bis(1-((3aR,4R,6aR)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)ethan-1-ol)" Molbank 2025, no. 1: M1962. https://doi.org/10.3390/M1962
APA StyleKadraoui, M., Guillarme, S., & Saluzzo, C. (2025). (1S,1′S)-2,2′-(Benzylazanediyl)bis(1-((3aR,4R,6aR)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)ethan-1-ol). Molbank, 2025(1), M1962. https://doi.org/10.3390/M1962





