Abstract
The synthesis of three biaryl systems containing the benzo[1,2-b:4,3-b’] framework was accomplished through the Suzuki–Miyaura cross-coupling reaction between 1-bromobenzo[1,2-b:4,3-b’]dithiophene and easily available polycyclic aromatic hydrocarbon boronic acid pinacol esters containing pyrene, fluorene, and fluorenone. The spectroscopic characterization of these molecules was carried out by means of NMR experiments and high-resolution mass spectrometry. UV-vis absorption measurements at different concentrations of the newly synthesized compounds were also performed.
1. Introduction
Benzo[1,2-b:4,3-b’]dithiophene (BDT) derivatives belong to the class of phenantrene-like tricyclic thiophene-containing compounds, which are widely studied in optoelectronics to build organic light-emitting diodes (OLEDs) [1], thin-film transistors (OFETs) [2,3], dyes for solar cells (DSSCs) [4,5,6,7], hole-transporting materials for perovskite solar cells (PSCs) [8,9], π-conjugated polymer semiconductors [10,11], copolymers for solar cells [12,13,14], and supramolecular structures [15,16]. The easy and regioselective functionalization of the BDT core allows the modulation of its physicochemical properties and makes BDT a valuable framework from which more complex and chiral heteroaromatic molecules can be prepared, including thiahelicenes and atropisomeric structures [17]. We recently reported an efficient two-step synthesis of 1-bromobenzo [1,2-b:4,3-b’]dithiophene (1) [18], which was used as starting material to obtain tetrathia[7]helicenes (7-THs) through the formation of bis(benzo[1,2-b:4,3-b’]dithiophene) 2 (Figure 1a). The latter compound was prepared in good yield (86%) by the palladium-catalyzed one-pot Miyaura borylation/Suzuki coupling of 1 in the presence of bis(pinacolato)diboron. In this work, we describe the synthesis and characterization of three novel BDT-based biaryl systems 3, which were obtained by Suzuki–Miyaura coupling between bromide 1 and polycyclic hydrocarbon boronic acid pinacol esters 4 (Figure 1b). Biaryls 3, in which the peculiar electronic and photophysical properties of pyrene [19], fluorene [20], and fluorenone [21] are combined with the electronic features inferred by the presence of sulfur atoms in the BDT skeleton, represent useful intermediates from which novel chiral molecules can be obtained.
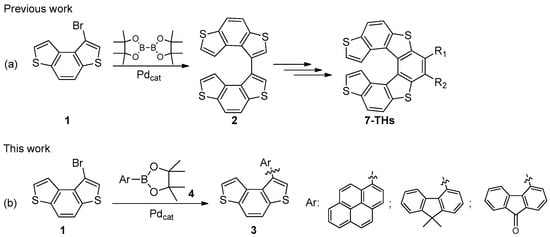
Figure 1.
(a) Synthesis of bis(benzodithiophene) 2 (previous work); (b) synthesis of biaryls 3 (this work).
2. Results and Discussion
2.1. Synthesis
The palladium-catalyzed Suzuki–Miyaura (SM) cross-coupling reaction is one of the most straightforward and efficient routes to synthesize biaryl compounds through the formation of the Csp2-Csp2 bond between the two aryl portions. Thus, we initially proposed two alternative SM reactions for the synthesis of BDT-based biaryls 3a–c in order to evaluate the outcome of this cross-coupling: (i) the Csp2-Csp2 bond formation between commercial bromides 5a–c and BDT-based boronic acid 6 or pinacol ester 7 (Scheme 1, route a); (ii) the Csp2-Csp2 bond formation between bromide 1 and boronic acid pinacol esters 4a–c (Scheme 1, route b).
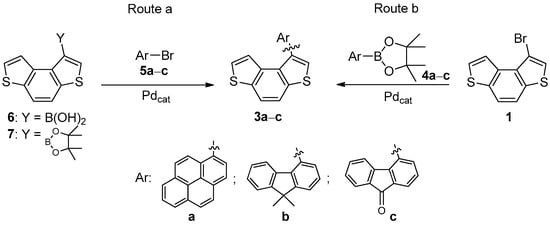
Scheme 1.
Two alternative routes towards the synthesis of biaryls 3a–c.
Regarding route a, the attempts to synthesize ester 7 by the palladium-catalyzed Miyaura borylation of bromide 1 failed under classical experimental conditions (i.e., Pd(dppf)Cl2, KOAc, dioxane), and unreacted bromide 1 and BDT along with traces of bis(benzodithiophene) 2 were obtained as the main products. Alternatively, the synthesis of boronic acid 6 was carried out through the Li/Br exchange reaction of 1 with nBuLi, followed by the reaction with triisopropyl borate and hydrolysis under acidic conditions, but the required product was not obtained.
Thus, to synthesize biaryls 3a–c, we focused our attention on route b, taking into account that ester 4b was commercially available, and that we had facile access to bromide 1 [18] and pinacol esters 4a [22] and 4c [23]. Both esters were prepared by the palladium-catalyzed Miyaura borylation of the corresponding commercial bromides 5a and 5c, according to previously reported methodologies.
In particular, the synthesis of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-fluoren-9-one (4c) was performed by the Miyaura borylation of commercial bromide 5c, following a similar procedure to that reported in the patent from Aventis Pharma [23] (Scheme 2).
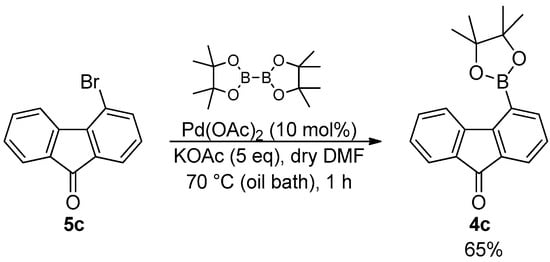
Scheme 2.
Miyaura borylation of 5c for the synthesis of ester 4c.
The desired ester 4c was isolated in 65% yield as a yellow powder after the chromatographic purification on silica gel of the crude reaction mixture. Since the characterization of 4c reported so far includes only 1H NMR and mass analysis, a complete NMR assignment of this compound was performed through 13C NMR and 2D methods.
We then proceeded with the synthesis of the target biaryl systems 3a–c through a Pd(PPh3)4-catalyzed SM reaction between bromide 1 and esters 4a–c (Scheme 3).
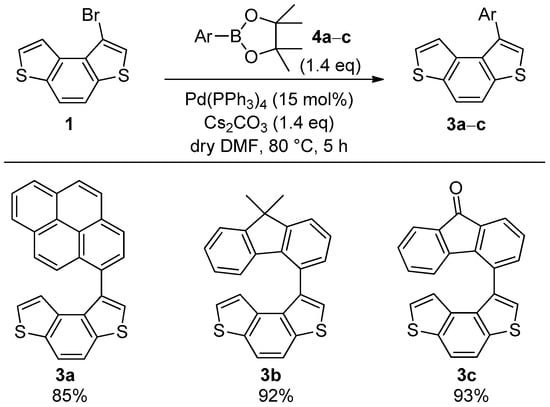
Scheme 3.
Synthesis of biaryls 3a–c through SM coupling between bromide 1 and esters 4a–c.
In particular, the reactions were carried out in the presence of Pd(PPh3)4 as a catalyst, and Cs2CO3 was used as a base in DMF at 80 °C. The required products 3a–c were isolated in good-to-excellent yields. These experimental conditions were very similar to those previously employed for the synthesis of benzothiophene-based biaryls [24], and were also found to be suitable for 3a–c, demonstrating the effectiveness of this method for diverse classes of thiophene-containing biaryl compounds.
2.2. UV-Vis Absorption Measurements
The UV-vis absorption of compounds 3a–c was measured in diluted solutions of DCM in a range of concentrations from 2 to 8 × 10–6 M. The UV-vis profile of each compound was not altered by the variation in concentration (see Figures S1–S3). Figure 2 shows the superimposed UV-vis spectra of 3a–c at 6 × 10–6 M, and Table 1 reports the selected absorption maxima values and the corresponding molar extinction coefficients for 3a–c.
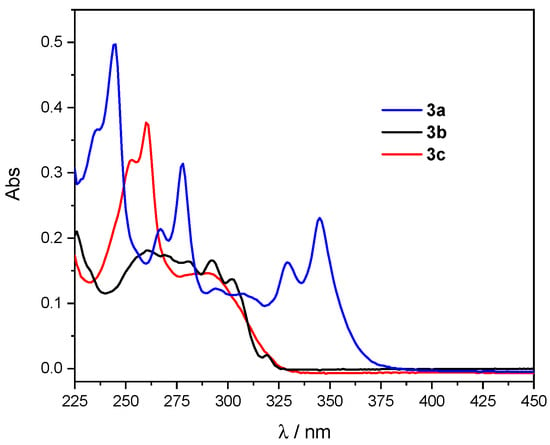
Figure 2.
Superimposed UV-vis spectra of 3a–c (DCM solutions at 6 × 10−6 M).

Table 1.
Selected absorption maxima and corresponding molar extinction coefficients for 3a–c.
The UV-vis absorption spectrum of 3a showed the typical resolved peaks of the pyrene moiety at around 245, 267, 278, 329, and 345 nm, with an absorption extending towards the visible region when compared to compounds 3b,c. This behavior was in agreement with the data previously obtained for the UV spectrum of 3-(pyren-1-yl)benzo[b]thiophene [24]. On the other hand, in the case of 3b and 3c, the absorption mainly occurred in the 250–300 nm region, with 3c displaying a distinct peak at 260 nm attributable to the fluorenone moiety.
These data demonstrate that the UV-vis absorption properties of the polycyclic hydrocarbon group in biaryls 3a–c were not significantly affected by the presence of the BDT core. Thus, these systems still present the peculiar properties of pyrene, fluorene, and fluorenone moieties, but also contain the BDT core; the proper functionalization of the core’s terminal thienyl rings could confer additional electronic and stereochemical features to 3a–c for innovative optoelectronics applications.
3. Materials and Methods
3.1. General Methods
All reactions were carried out under an inert atmosphere by means of standard techniques for manipulating air-sensitive compounds. Reagents and solvents obtained from commercial sources were used as received. 2-(9,9-Dimethyl-9H-fluoren-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (4b) and 4-bromo-9H-fluoren-9-one (5c) were purchased from TCI. Anhydrous DMF was purchased from Sigma-Aldrich (100 mL bottle with crown cap). 1-Bromobenzo[1,2-b:4,3-b’]dithiophene (1) [18] and 4,4,5,5-tetramethyl-2-(pyren-1-yl)-1,3,2-dioxaborolane (4a) [22] were synthesized according to the literature. The outcome of the reactions was monitored by thin-layer chromatography (TLC) on silica gel 60 F254 precoated plates, and plates were visualized with short-wave UV light (254 and 366 nm). Column chromatography was performed on silica gel 60 (70–230 mesh).
The 1H and 13C{1H} NMR spectra of compounds 3a–c were identified on Bruker (Berlin, Germany) AC-300 and Avance 600 MHz spectrometers, while the NMR experiments for compound 4c were recorded on a Bruker DRX-400 MHz instrument. The chemical shifts are given in ppm (δ) relative to the residual protonated solvent resonances and coupling constants in Hz. High-resolution mass spectra (HRMS) were recorded with an electron ionization (EI) spectrometer FISONS–Vg Autospec- M246 (Fisons Instruments, Glasgow, GB).
UV-vis absorption spectra were recorded on a Shimadzu (Kyoyo, Japan) UV3600 spectrophotometer using quartz cuvettes with a 1 cm optical path length.
3.2. Synthesis of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-fluoren-9-one (4c)
A degassed mixture of bromide 5c (200 mg, 0.77 mmol), bis(pinacolato)diboron (479 mg, 1.88 mmol), Pd(OAc)2 (17 mg, 0.077 mmol), and KOAc (378 mg, 3.86 mmol) in dry DMF (15 mL) was stirred at 70° C (oil bath) under an argon atmosphere for 1 h. The mixture was then cooled to room temperature, diluted with DCM (10 mL), and poured into water (50 mL). The aqueous phase was extracted with DCM (3 × 20 mL), and the collected organic phases were washed with water (4 × 20 mL), dried over Na2SO4, and concentrated under reduced pressure. The crude mixture was purified by column chromatography on silica gel (eluent: hexane/ethyl acetate, 98/2) to afford 4c as a yellow solid (153 mg, 65%). 1H NMR (400 MHz, DMSO-d6) δ: 8.48 (d, J = 7.5 Hz, 1H, H11), 7.88 (dd, J = 7.5, 1.2 Hz, 1H, H5), 7.73 (dd, J = 7.3, 1.2 Hz, 1H, H3), 7.66–7.61 (m, 2H, H12 and H14), 7.43–7.37 (m, 2H, H4 and H13), 1.41 (s, 12H, H8). 13C NMR (100 MHz, DMSO-d6) δ: 193.1 (C1), 148.6 (C9), 144.8 (C10), 142.2 (C5), 135.3 (C12), 133.5 (C2 and C15), 129.5 (C13), 128.4 (C4), 126.2 (C3), 124.4 (C11), 123.6 (C14), 84.4 (2 C7), 24.6 (4 C8) (See Figure 3).
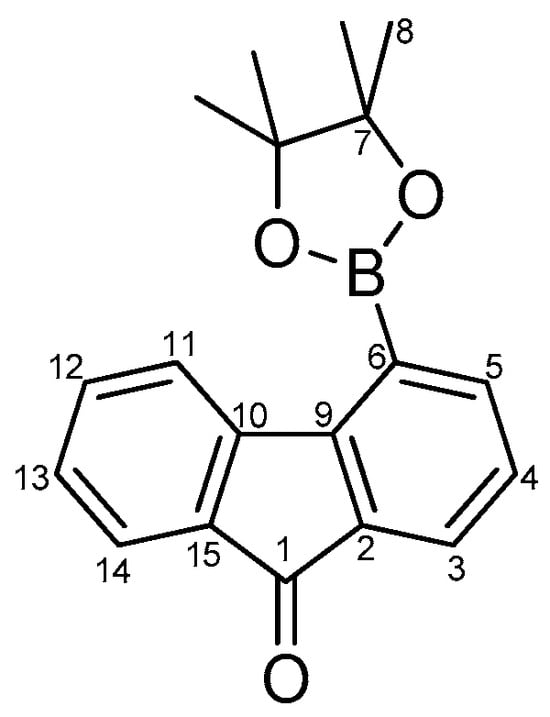
Figure 3.
Numbering of intermediate 4c for the NMR assignment.
3.3. General Procedure for the Synthesis of Biaryls 3a–c
To a flame-dried vessel, bromide 1 (55 mg, 0.20 mmol), ester 4a–c (0.28 mmol), Pd(PPh3)4 (42 mg, 0.036 mmol), and Cs2CO3 (91 mg, 0.28 mmol) were added. The reaction vessel was fitted with a silicon septum, evacuated, and back-filled with argon, and this sequence was repeated twice. Dry DMF (1 mL, 0.2 M) was added, and the mixture was stirred at 80 °C (oil bath) for 5 h under an argon atmosphere. After cooling to room temperature, the mixture was diluted with DCM (10 mL) and poured into water (20 mL). The aqueous phase was extracted with DCM (3 × 10 mL), and the collected organic phases were washed with water (2 × 10 mL) and dried over Na2SO4. The solvent was removed under reduced pressure, and the crude mixture was purified by chromatography on silica gel to provide compounds 3a–c.
3.3.1. 1-(Pyren-1-yl)benzo [1,2-b:4,3-b’]dithiophene (3a)
The crude product obtained from the SM reaction of bromide 1 with ester 4a was purified by column chromatography on silica gel (eluent: hexane/DCM, 95/5) to afford 3a as a pink powder (66 mg, 85%). 1H NMR (600 MHz, CDCl3): δ = 8.30 (d, J = 7.7 Hz, 1H), 8.25 (d, J = 7.6 Hz, 1H), 8.21–8.16 (m, 2H), 8.15 (d, J = 7.5 Hz, 1H), 8.09 (d, J = 7.7 Hz, 1H), 8.04 (t, J = 7.6 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H), 7.92 (d, J = 8.8 Hz, 1H), 7.90 (d, J = 9.2 Hz, 1H), 7.81 (d, J = 9.2 Hz, 1H), 7.60 (s, 1H), 6.94 (d, J = 5.6 Hz, 1H), 6.13 ppm (d, J = 5.6 Hz, 1H). 13C NMR (150 MHz, CDCl3): δ = 137.7 (Cq), 137.2 (Cq), 137.0 (Cq), 134.7 (Cq), 134.5 (Cq), 132.7 (Cq), 131.6 (Cq), 131.5 (Cq), 131.2 (Cq), 130.5 (Cq), 128.4 (CH), 128.1 (CH), 128.0 (CH), 127.6 (CH), 126.3 (CH), 125.8 (CH), 125.65 (CH), 125.57 (CH), 125.4 (2CH), 124.9 (2Cq), 124.8 (CH), 122.1 (CH), 119.5 (CH), 119.2 ppm (CH). HRMS-EI: calcd. for C26H14S2 [M]+ 390.0536, found 390.0540.
3.3.2. 1-(9,9-Dimethyl-9H-fluoren-4-yl)benzo [1,2-b:4,3-b’]dithiophene (3b)
The crude product obtained from the SM reaction of bromide 1 with ester 4b was purified by column chromatography on silica gel (eluent: hexane/DCM, 9/1) to afford 3b as a colorless powder (70 mg, 92%). 1H NMR (600 MHz, CDCl3): δ = 7.92 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 8.7 Hz, 1H), 7.60 (dd, J = 7.5, 1.1 Hz, 1H), 7.50 (s, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.39–7.36 (m, 1H), 7.33 (dd, J = 7.4, 1.0 Hz, 1H), 7.16–7.10 (m, 2H), 6.82 (td, J = 7.8, 1.1 Hz, 1H), 6.57 (d, J = 5.6 Hz, 1H), 6.43 (d, J = 7.7 Hz, 1H), 1.57 ppm (s, 6H). 13C NMR (150 MHz, CDCl3): δ = 154.5 (Cq), 153.9 (Cq), 138.8 (Cq), 138.3 (Cq), 137.5 (Cq), 137.2 (Cq), 136.9 (Cq), 134.9 (Cq), 133.6 (Cq), 132.2 (Cq), 129.7 (CH), 127.2 (CH), 127.1 (CH), 127.0 (CH), 125.8 (CH), 123.9 (CH), 122.7 (CH), 122.6 (CH), 122.3 (CH), 121.7 (CH), 119.4 (CH), 119.2 (CH), 46.6 (Cq), 28.1 (CH3), 27.2 ppm (CH3). HRMS-EI: calcd. for C25H18S2 [M]+ 382.0850, found 382.0847; [M–CH3]+ 367.1128.
3.3.3. 4-(Benzo [1,2-b:4,3-b’]dithiophen-1-yl)-9H-fluoren-9-one (3c)
The crude product obtained from the SM reaction of bromide 1 with ester 4c was purified by column chromatography on silica gel (eluent: hexane/ethyl acetate, 98/2) to afford 3c as a yellow powder (69 mg, 93%). 1H NMR (600 MHz, CDCl3): δ = 7.94–7.89 (m, 2H), 7.86–7.83 (m, 1H), 7.62 (d, J = 7.3 Hz, 1H), 7.51 (s, 1H), 7.50–7.47 (m, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.27–7.25 (m, 1H), 7.11 (t, J = 7.4 Hz, 1H), 6.99 (t, J = 7.6 Hz, 1H), 6.87 (d, J = 5.6 Hz, 1H), 6.30 ppm (d, J = 7.7 Hz, 1H). 13C NMR NMR (75 MHz, CDCl3): δ = 193.9 (Cq), 144.2 (Cq), 143.2 (Cq), 137.8 (Cq), 137.5 (CH), 137.4 (Cq), 135.0 (CH), 134.9 (2Cq), 134.6 (Cq), 134.4 (Cq), 132.9 (2Cq), 129.2 (CH), 129.0 (CH), 126.6 (CH), 124.7 (CH), 124.34 (CH), 124.27 (CH), 122.9 (CH), 121.4 (CH), 119.8 (CH), 119.2 ppm (CH). HRMS-EI: calcd. for C23H12OS2 [M]+ 368.0329, found 368.0330.
4. Conclusions
The synthesis of three novel BDT-based biaryl systems 3a–c containing a pyrene, a fluorene, and a fluorenone moiety was reported through efficient SM coupling, starting from easily available bromide 1 and boronic acid pinacol esters 4a–c. Preliminary UV-vis absorption measurements in diluted solutions of these compounds showed that the peculiar optical properties of the polycyclic hydrocarbon group were preserved in the biaryl system, thus providing novel and enhanced functional organic compounds. Indeed, the proper modification and functionalization of these biaryls, especially exploiting the reactivity of the terminal thiophene rings, could lead to a novel class of inherently chiral atropisomers and/or thiahelicenes with potential applications in different domains (e.g., non-linear optical materials, asymmetric synthesis, and enantioselective electroanalysis).
Supplementary Materials
NMR spectra of 3a–c and 4c; HRMS-EI spectra of 3a–c; UV-vis absorption spectra of 3a–c at different concentrations.
Author Contributions
Conceptualization, S.C. and V.P.; methodology, V.P, L.F. and F.F.; data curation, V.P and F.F.; writing—original draft preparation, S.C., V.P. and A.C.; writing—review and editing, S.C., V.P., A.C. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
V.P. thanks Università degli Studi di Milano for the Ph.D. fellowship. NMR analyses were performed at the NMR facility of the Unitech COSPECT at the Università degli Studi di Milano.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bossi, A.; Arnaboldi, S.; Castellano, C.; Martinazzo, R.; Cauteruccio, S. Benzodithienyl Silanes for Organic Electronics: AIE Solid-State Blue Emitters and High Triplet Energy Charge-Transport Materials. Adv. Optical Mater. 2020, 8, 2001018. [Google Scholar] [CrossRef]
- Kim, C.; Marks, T.J.; Facchetti, A.; Schiavo, M.; Bossi, A.; Maiorana, S.; Licandro, E.; Todescato, F.; Toffanin, S.; Muccini, M. Synthesis, characterization, and transistor response of tetrathia-[7]-helicene precursors and derivatives. Org. Electron. 2009, 10, 1511–1520. [Google Scholar] [CrossRef]
- Akimoto, I.; Kan’no, K.-i.; Osuga, H.; Tanaka, K. Photo-luminescence properties and exciton–carrier interaction in 1,2-diarylethylene derivatives films. J. Lumin. 2005, 112, 341–344. [Google Scholar] [CrossRef]
- Li, X.; Wu, K.; Zheng, L.; Deng, Y.; Tan, S.; Chen, H. Synthesis and characterization of novel benzodithiophene-fused perylene diimide acceptors: Regulate photovoltaic performance via structural isomerism. Dyes Pigments 2019, 168, 59–67. [Google Scholar] [CrossRef]
- Lu, X.; Lan, T.; Qin, Z.; Wang, Z.-S.; Zhou, G. A Near-Infrared Dithieno[2,3-a:3′,2′-c]phenazine-Based Organic Co-Sensitizer for Highly Efficient and Stable Quasi-Solid-State Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 19308–19317. [Google Scholar] [CrossRef]
- Longhi, E.; Bossi, A.; Di Carlo, G.; Maiorana, S.; De Angelis, F.; Salvatori, P.; Petrozza, A.; Binda, M.; Roiati, V.; Mussini, P.R. Metal-Free Benzodithiophene-Containing Organic Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2013, 2013, 84–94. [Google Scholar] [CrossRef]
- Gao, P.; Tsao, H.N.; Grätzel, M.; Nazeeruddin, M.K. Fine-tuning the Electronic Structure of Organic Dyes for Dye-Sensitized Solar Cells. Org. Lett. 2012, 14, 4330–4333. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Shen, C.; Zhang, D.; Liu, S.; Wu, Y.; Zhu, W.-H. A Coplanar π-Extended Quinoxaline Based Hole-Transporting Material Enabling over 21 % Efficiency for Dopant-Free Perovskite Solar Cells. Angew. Chem. Int. Ed. 2021, 60, 2674–2679. [Google Scholar] [CrossRef]
- Cui, B.-B.; Yang, N.; Shi, C.; Yang, S.; Shao, J.-Y.; Han, Y.; Zhang, L.; Zhang, Q.; Zhong, Y.-W.; Chen, Q. Naphtho[1,2-b:4,3-b′]dithiophene-based hole transporting materials for high-performance perovskite solar cells: Molecular engineering and opto-electronic properties. J. Mater. Chem. A 2018, 6, 10057–10063. [Google Scholar] [CrossRef]
- Li, C.; Zheng, N.; Chen, H.; Huang, J.; Mao, Z.; Zheng, L.; Weng, C.; Tan, S.; Yu, G. Synthesis, characterization, and field-effect transistor properties of tetrathienoanthracene-based copolymers using a two-dimensional π-conjugation extension strategy: A potential building block for high-mobility polymer semiconductors. Polym. Chem. 2015, 6, 5393–5404. [Google Scholar] [CrossRef]
- Bedi, A.; Zade, S.S. Electrochemical Route to Solution-Processable Polymers of Thiophene/Selenophene Capped Didodecyloxybenzo[1,2-b:4,3-b′]dithiophene and Their Optoelectronic Properties. Macromolecules 2013, 46, 8864–8872. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W.; Qin, H.; Yi, X.; Zeng, W.; Zhao, Y.; Chen, H. Electron-Transporting Conjugated Polymers from Novel Aromatic Five-Membered Diimides: Naphtho[1,2-b:4,3-b′]-dithiophene and -Diselenophene Diimides. Macromolecules 2023, 56, 2990–3003. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Konstantinov, I.O.; Khokhlov, A.R.; Ostapov, I.E.; Godovsky, D.Y.; Alekseev, V.G.; Zou, Y.; Singhal, R.; Singh, M.K.; Sharma, G.D. New Wide Bandgap Conjugated D-A Copolymers Based on BDT or NDT Donor Unit and Anthra[1,2-b:4,3,bʹ:6,7-cʺ]trithiophene-8-12-dione Acceptor for Fullerene-Free Polymer Solar Cells. Macromol. Chem. Phys. 2022, 223, 2200168. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Guo, X.; Cui, C.; He, Y.; Li, Y. Synthesis and Characterization of Dioctyloxybenzo[1,2-b:4,3-b′]dithiophene-Containing Copolymers for Polymer Solar Cells. Macromolecules 2011, 44, 7625–7631. [Google Scholar] [CrossRef]
- Fritzsche, M.; Bohle, A.; Dudenko, D.; Baumeister, U.; Sebastiani, D.; Richardt, G.; Spiess, H.W.; Hansen, M.R.; Höger, S. Empty Helical Nanochannels with Adjustable Order from Low-Symmetry Macrocycles. Angew. Chem. Int. Ed. 2011, 50, 3030–3033. [Google Scholar] [CrossRef] [PubMed]
- Jester, S.-S.; Sigmund, E.; Höger, S. Nanopatterning by Molecular Polygons. J. Am. Chem. Soc. 2011, 133, 11062–11065. [Google Scholar] [CrossRef] [PubMed]
- Cauteruccio, S.; Dreuw, A.; Licandro, E.; Mussini, P.R. Tetrathiahelicenes: An Infinite Source of Inspiration. In Helicenes: Synthesis, Properties and Applications; Crassous, J., Stará, I.G., Starý, I., Eds.; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar] [CrossRef]
- Pelliccioli, V.; Dova, D.; Baldoli, C.; Graiff, C.; Licandro, E.; Cauteruccio, S. Diversified Syntheses of Tetrathia[7]helicenes by Metal-Catalyzed Cross-Coupling Reactions. Eur. J. Org. Chem. 2021, 2021, 383–395. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Shaya, J.; Corridon, P.R.; Al-Omari, B.; Aoudi, A.; Shunnar, A.; Mohideen, M.I.H.; Qurashi, A.; Michel, B.Y.; Burger, A. Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: A review. J. Photochem. Photobiol. C Photochem. Rev. 2022, 52, 100529. [Google Scholar] [CrossRef]
- Neha, N.K. Unveiling the versatile applications of 9-fluorenone: A comprehensive review 2010–2024. Coord. Chem. Rev. 2024, 521, 216173. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Zhang, K.; Dai, J.; Bai, W.; Bai, R. Pyrene boronic acid cyclic ester: A new fast self-recovering mechanoluminescent material at room temperature. Chem. Commun. 2016, 52, 9679–9682. [Google Scholar] [CrossRef] [PubMed]
- Mailliet, P.; Bertin, L.; Thompson, F.; Ruxer, J.M.; Pilorge, F.; Benard, D.; Minoux, H.; Carrez, C.; Goulaouic, H.; Gouyon, T.; et al. Novel Fluorene Derivatives, Composition Containing Said Derivatives and the Use Thereof. U.S. Patent 7,674,795.2007.
- Pelliccioli, V.; Cardano, F.; Renno, G.; Vasile, F.; Graiff, C.; Mazzeo, G.; Fin, A.; Longhi, G.; Abbate, S.; Rosetti, A.; et al. Synthesis, Stereochemical and Photophysical Properties of Functionalized Thiahelicenes. Catalysts 2022, 12, 366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).