Abstract
3-N-allylrhodanine was condensed with 5-(4-methoxyphenyl)-thiophene-2-carbaldehyde in an L-proline-based deep eutectic solvent (DES) to obtain the π-conjugated heterocyclic rhodanine compound (5Z)-3-allyl-5-{[5-(4-methoxyphenyl)thiophen-2-yl]methylidene}-2-sulfanylidene-1,3-thiazolidin-4-one (2). Compound 2 was characterized by NMR spectroscopy, and its UV-vis spectrum was compared with that of the related derivative 3-allyl-5-(4-methoxybenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one (1). Preliminary results revealed that compound 2 is emissive at room temperature in solution.
1. Introduction
Rhodanines, thiazolidinediones, thiazolidinones and barbituric derivatives are very interesting heterocyclic compounds, and have been studied for a long time, as they present a number of pharmacological activities [1,2]. A review that recently analyzed the anticancer features of the rhodanines described over the last decade in the literature discusses the structure–activity relationship of those rhodanine derivatives [3]. Especially, the authors focused on substituents introduced in positions 3 and 5, trying to push forward further development of rational drug design. For example, analysis of the literature results suggests that the presence of a heteroaryl moiety in position 5 may allow better anticancer activity [3]. Rhodanine derivatives also display many other biological activities, such as inhibiting human carbonic anhydrase [4], α-amylase and α-glucosidase inhibitors [5], and as antihyperglycemic [6] or antimicrobial agents [7]. We previously described 2-(heteroarylimino)-1,3-thiazolidin-4-ones having an antiproliferative effect against human colon and breast cancer-derived cells [8] or being CDC25A inhibitors [9]. More recently, we worked on the functionalization of rhodanines via Knoevenagel reaction in an L-proline-based DES instead of employing a conventional Knoevenagel condensation protocol using organic solvents as the reaction medium. Some of the synthesized 5-arylidenerhodanines showed interesting antioxidant properties [10]. We then extended this zero-VOC strategy for Knoevenagel reaction to thiazolidine-2,4-dione and barbituric acid [11].
We present here the synthesis of the hitherto unknown 5-arylidenerhodanine 2 obtained from 5-(4-methoxyphenyl)-thiophene-2-carbaldehyde. This compound presents similarities with rhodanine-3-acetic acid derivative A, which was studied as a fluorescent probe for neurofibrillary tangles in Alzheimer’s disease brains [12] (Figure 1). It is also related to donor–acceptor compounds, such as B, described in 2020 in the search for new thiophene-based dyes for organic metal-free dye-sensitized solar cells [13].
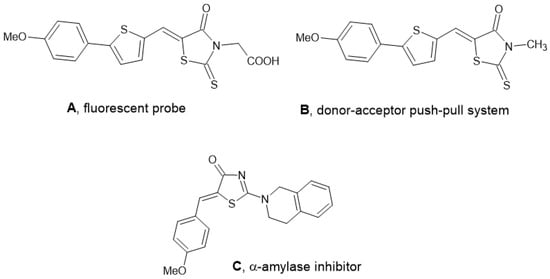
Figure 1.
Examples of rhodanines displaying biological or photophysical activities.
So, compound 2 may be interesting for biological activities or for photoelectronic applications, but can also be studied by coordination chemists, as the C=S thione function present in rhodanine may coordinate with transition metal and form Cu(I) or Ag(I) complexes [14]. A nucleophilic addition of 1,2,3,4-tetrahydroisoquinoline (THIQ) may also take place on the thione function giving 2-aminothiazol-4(5H)-one similar to derivative C, described as an α-amylase inhibitor (Figure 1) [15].
2. Results and Discussion
Recently, we showed that L-proline-based DESs were very powerful when performing Knoevenagel condensations in short reaction times with high yields and without any purification [10,11]. However, the DES Pro:Gly (1:2) we previously used is quite viscous at ambient temperature [16], and, therefore, is sometimes not very easy to handle. Particularly, in some cases with certain substrates, agitation became very difficult over time when precipitation occurred in the reaction media. So, we turned our attention to other L-proline-based DESs that are less viscous.
5-(4-Methoxyphenyl)-thiophene-2-carbaldehyde was synthesized according to the reported procedure [17] and engaged in Knoevenagel condensation with N-allylrhodanine in Pro:EG (1:20). After 3 h at 80 °C, the reaction workup only involved the addition of water for separating the targeted product from the aqueous phase containing the DES. Derivative 2 was obtained in pure form at a 96% yield, and no further purification was needed (Scheme 1).

Scheme 1.
Synthesis of targeted rhodanine 2.
The 1H-NMR spectrum (Figure 2) was in agreement with the reported spectra of compounds A and B [12,13]. Especially, the vinylic proton appeared at δ 8.07 ppm, which indicates a Z-configuration for the exocyclic double bond, as usually observed in 5-arylidene rhodanines [18,19]. Camero et al. have shown that a Z/E photoisomerization is possible on 2-(1,1-dicyanomethylene)rhodanines functionalized with oligothiophenes [20]. In this case, the vinylic proton appearing at δ 8.0–8.15 ppm in the Z-form was offset to δ 7.30–7.45 ppm in the E-form [20].
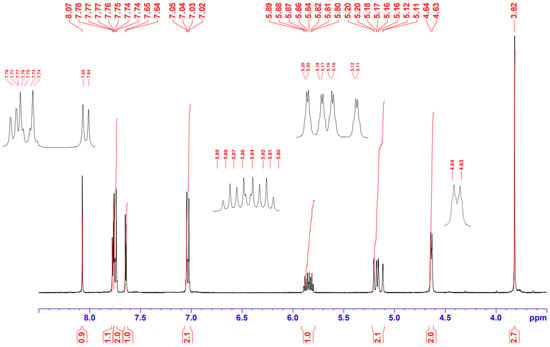
Figure 2.
1H NMR spectrum (400 MHz, DMSO-d6) of derivative 2.
The thiophene ring gave rise to two doublets at δ 7.64 and 7.77 ppm with a 3J coupling of 3.8 Hz, whereas the aromatic protons appeared at δ 7.03 and 7.75 ppm with a 3J coupling of 8.3 Hz. Signals of the protons in the allyl group were observed between 4.63 and 5.89 ppm and were consistent with reported data on N-allylrhodanines [21,22]. A pseudo doublet of triplets appeared at δ 4.63 ppm for the NCH2, two doublets of doublets could be seen at δ 5.19 and 5.13 ppm for the terminal vinylic protons (with Jtrans = 17.0 Hz, Jcis = 10.1 Hz and Jgem = 1.4 Hz), and the signal at δ 5.84 ppm corresponded to the internal vinylic proton.
The 13C{1H} NMR also showed characteristic signals of the N-allyl group (CH2 at δ 46.2 ppm and =CH2 at δ 118.5 ppm) and rhodanine moiety (C=S at δ 191.8 and C=O at δ 166.3 ppm). The structure of compound 2 was also confirmed by HRMS analysis (see Figures S3 and S4 in Supplementary Materials).
Finally, the UV-vis spectrum of highly π-conjugated 2 bearing a thiophene linker was compared to that of 3-allyl-5-(4-methoxybenzylidene)-2-thioxothiazolidin-4-one 1 (Figure 3). This literature-known compound [22,23] had already been synthesized in our former study [21]. Firstly, the introduction of a thiophene moiety between the N-allylrhodanin and anisole fragments induced an increase in the molar extinction coefficients of all bands (Table 1). Secondly, there was a bathochrome effect of about 23 nm for the first band and, which is remarkable, of 59 nm for the second band. This bathochromic shift was due to the extension of the conjugation that may result in a decrease in the energy difference between the HOMO and LUMO orbitals.
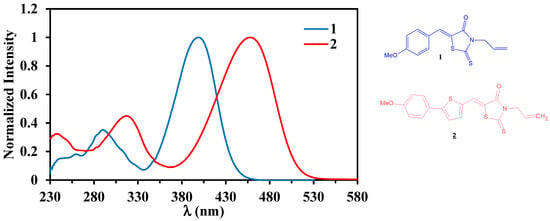
Figure 3.
Superposition of normalized absorption spectra recorded for 1 and 2 in CH2Cl2 at rt.

Table 1.
Absorption data of compounds 1 and 2 in CH2Cl2 at rt.
During this study, we were also interested in the luminescence properties of compound 2 in solution. After excitation at 450 nm, an intense emission band was observed (Figure 4) with a maximum at 535 nm. The fluorescence quantum yield ØF of 2 equaled 31%, and was determined using pyrene (ØF = 27%) in CH2Cl2 as a luminescence quantum yield standard [24]. This fluorescence band can be assigned to the lowest energy singlet state S1→S0 transition. To understand more in depth the contribution of the orbitals involved in the observed emission, detailed photophysical studies and theoretical calculations regarding this compound and other molecules of this family are underway.
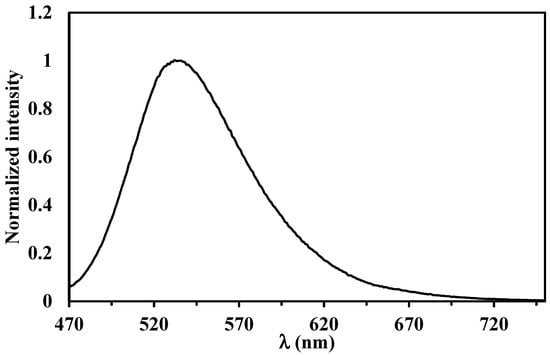
Figure 4.
Normalized emission of 2 recorded in CH2Cl2 at room temperature.
In conclusion, we synthesized and fully characterized the new derivative 2, which may be interesting for biological study or for further functionalization. Finally, studying luminescence properties of 2 revealed its fluorescence in solution at room temperature.
3. Materials and Methods
All reactions were routinely checked by TLC analysis on an Alugram SIL G/UV254 (Macherey-Nagel) with spots visualized by UV light. The melting point was determined in open capillaries on a Stuart SMP30 apparatus (Bibby Scientific Ltd, Staffordshire, United-Kingdom) and was uncorrected. The 1H and 13C-NMR spectra were measured on an AC Bruker 400 MHz spectrometer (Bruker, Wissembourg, France) in DMSO-d6; chemical shifts are reported in parts per million (ppm). All coupling constants (J) are given in Hz. UV-vis spectra were obtained on a VARIAN-Cary 300 array spectrophotometer (Varian, Melbourne, Australia). The IR spectrum was obtained on a ThermoScientific Nicolet IS5 apparatus (ThermoScientific, Madison, WI, USA).
5-(4-Methoxyphenyl)-thiophene-2-carbaldehyde was prepared according to the published method [17].
Formation of DES L-Proline:Ethylene Glycol (1:20)
In a round-bottomed flask, the hydrogen bond donor and hydrogen bond acceptor were mixed in the appropriate molar ratio and stirred at 60 °C until a clear solution formed. The DES was then used without further purification.
General procedure for the Knoevenagel condensation
A total of 2 g of DES was introduced in a 10 mL round-bottom flask. Next, aldehyde (1 mmole) and N-allylrhodanine (1 mmole) were sequentially added to the DES, and the reaction mixture was stirred at 80 °C for 3 h. Water was added at room temperature and the precipitate was collected by filtration and washed with 15 mL of water. No further purification was needed.
(5Z)-3-Allyl-5-{[5-(4-methoxyphenyl)thiophen-2-yl]methylidene}-2-sulfanylidene-1,3-thiazolidin-4-one 2. Red solid (348 mg yield = 96%). M.p.: 149 °C. 1H NMR (DMSO-d6): δ 3.81 (s, 3H, OCH3), 4.64 (td, J = 5.6 and 1.4 Hz, 2H, NCH2), 5.13 (dd, J = 17.0 and 1.4 Hz, 1H, =CH2), 5.19 (dd, J = 10.1 and 1.4 Hz, 1H, =CH2), 5.84 (ddt, J = 10.1, 17.0 and 5.6 Hz, 1H, =CH), 7.03 (d, J = 8.3 Hz, 2H, Ar-H), 7.64 (d, J = 3.8 Hz, 1H, H-thiophene), 7.75 (d, J = 8.3 Hz, 2H, Ar-H), 7.77 (d, J = 3.8 Hz, 1H, H-thiophene), 8.07 (s, 1H, =CH). 13C NMR (DMSO-d6): δ 46.2, 55.4, 114.8, 117.8, 118.5, 124.7, 125.2, 126.4, 127.5, 130.3, 135.5, 138.0, 152.3, 160.3, 166.3, 191.8. HRMS LDI (-) FT-ICR: m/z [M]- calcd for [C18H15NO2S3]●−: 373.027041; found: 373.027077. IR-ATR: 1698 ν(C=O), 1205 ν(C=S) cm−1.
Supplementary Materials
The following supporting information are available online: Figure S1. 1H NMR spectrum (400 MHz, DMSO-d6) of derivative 2; Figure S2. 13C NMR spectrum (100 MHz, DMSO-d6) of derivative 2; Figure S3. LDI (-) FT-ICR mass spectrum achieved for sample 2 with [C18H15NO2S3]●− ion at m/z 373.027077. Figure S4. Experimental (red) and theoretical (black) isotopic distributions for [C18H15NO2S3]●− ion achieved by LDI (-) FT-ICR MS. Figure S5. IR spectrum of derivative 2.
Author Contributions
S.H. prepared the compound; A.K. obtained UV-vis and emission spectra; S.H., I.J., A.K. and M.K. designed this study, analyzed data and wrote this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors acknowledge the NMR platform of the University of Lorraine, especially Sandrine Rup-Jacques and the MassLor platform and Jasmine Hertzog.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tomasic, T.; Masic, L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef] [PubMed]
- Mendgen, T.; Steuer, C.; Klein, C.D. Privileged scaffolds or promiscuous binders: A comparative study on rhodanines and related heterocycles in medicinal chemistry. J. Med. Chem. 2012, 55, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, J.; Tuszewska, H.; Trotsko, N. Anticancer profile of rhodanines: Structure-activity relationship (SAR) and molecular targets—A review. Molecules 2022, 27, 3750. [Google Scholar] [CrossRef]
- Yararli, K.; Ozer, E.B.; Bayindir, S.; Caglayan, C.; Turkes, C.; Beydemir, S. The synthesis, biological evaluation and in silico studies of asymmetric 3,5-diaryl-rhodanines as novel inhibitors of human carbonic anhydrase isoenzymes. J. Mol. Struct. 2023, 1276, 134783. [Google Scholar] [CrossRef]
- Srinivasa, M.G.; Aggarwal, N.N.; Gatpoh, B.F.D.; Shankar, M.K.; Ravindranath, K.B.; Veeranna, P.G.; Dixit, S.; Mandal, S.P.; Ravanappa, P.K.B.; Khanal, P.; et al. Identification of benzothiazole-rhodanine derivatives as α-amylase and α-glucosidase inhibitors: Design, synthesis, in silico and in vitro analysis. J. Mol. Recognit. 2022, 35, e2959. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.P.; Reji, A.; Bhavimani, G.; Prabitha, P.; Durai, P.; Yuvaraj, S.; Shashank, A.; Krishna, K.L.; Kumar, B.R.P. Rational design, synthesis and evaluation of novel rhodanine derivatives for antihyperglycemic activity. Polycycl. Aromat. Compd. 2022, 42, 1794–1805. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Chandrasekaran, B.; Palkar, M.B.; Kanhed, A.M.; Kajee, A.; Mlisana, K.P.; Singh, P.; Ghai, M.; Mahlalela, M.C.; Karpoormath, R. Synthesis and biological evaluation of novel carbazole hybrids as promising antimicrobial agents. Chem. Biodivers. 2020, 17, e1900550. [Google Scholar] [CrossRef]
- Revelant, G.; Huber-Villaume, S.; Dunand, S.; Kirsch, G.; Schohn, H.; Hesse, S. Synthesis and biological evaluation of novel 2-heteroarylimino-1,3-thiazolidin-4-ones as potential anti-tumor agents. Eur. J. Med. Chem. 2015, 94, 102–112. [Google Scholar] [CrossRef]
- Huber-Villaume, S.; Revelant, G.; Sibille, E.; Philippot, S.; Morabito, A.; Dunand, S.; Chaimbault, P.; Bagrel, D.; Kirsch, G.; Hesse, S.; et al. 2-(Thienylthiazolylimino)-1,3-thiazolidin-4-ones inhibit cell division cycle 25A phosphatase. Bioorg. Med. Chem. 2016, 24, 2920–2928. [Google Scholar] [CrossRef]
- Hesse, S. Synthesis of 5-arylidenethodanines in L-proline-based deep eutectic solvent. Beilstein J. Org. Chem. 2023, 19, 1537–1544. [Google Scholar] [CrossRef]
- Hesse, S.; Hertzog, J.; Rup-Jacques, S. L-proline-based DES in Knoevenagel synthesis of arylidene rhodanines, thiazolidine-2,4-diones and barbituric derivatives. J. Heterocycl. Chem. 2024, 61, 1015–1023. [Google Scholar] [CrossRef]
- Anumala, U.R.; Gu, J.; Lo Monte, F.; Kramer, T.; Heyny-von Haußen, R.; Hölzer, J.; Goetschy-Meyer, V.; Schön, C.; Mall, G.; Hilger, I.; et al. Fluorescent rhodanine-3-acetic acids visualized neurofibrillary tangles in Alzheimer’s disease brains. Biorg. Med. Chem. 2013, 21, 5139–5144. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Daniel, S.; Müller, T.J.J. Diversity-oriented approach to functional thiophene dyes by Suzuki coupling-lithiation one-pot sequences. Org. Chem. Front. 2020, 7, 329–339. [Google Scholar] [CrossRef]
- Hameau, A.; Guyon, F.; Khatyr, A.; Knorr, M.; Strohmann, C. 4,5-Bis(Methylthio)-1,3-Dithiole-2-Thione, a Versatile Sulphur-Rich Building Block for the Self-Assembly of Cu(I) and Ag(I) Coordination Polymers: Dithioether versus Thiocarbonyl Bonding. Inorg. Chim. Acta 2012, 388, 60–70. [Google Scholar] [CrossRef]
- Toumi, A.; Boudriga, S.; Hamden, K.; Sobeh, M.; Cheurfa, M.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L. Synthesis, Antidiabetic Activity and Molecular Docking Study of Rhodanine-Substitued Spirooxindole Pyrrolidine Derivatives as Novel α-Amylase Inhibitors. Bioorg. Chem. 2021, 106, 104507. [Google Scholar] [CrossRef]
- Meredith, L.; Elbourne, A.; Greaves, T.L.; Bryant, G.; Bryant, S.J. Physico-chemical characterisation of glycerol- and ethylene glycol-based deep eutectic solvents. J. Mol. Liquids 2024, 394, 123777. [Google Scholar] [CrossRef]
- Costa, S.P.G.; Batista, R.M.F.; Cardoso, P.; Belsley, M.; Raposo, M.M.M. 2-arylthienyl-substituted 1,3-benzothiazoles as new nonlinear optical chromophores. Eur. J. Org. Chem. 2006, 17, 3938–3946. [Google Scholar] [CrossRef]
- De Dios, A.; de la Puente, M.L.; Rivera-Sagredo, A.; Espinosa, J.R. Structural and conformational studies of 5-(1H-pyrrol-2-ylmethylene)-substituted imidazolidine-2,4-diones and thiazolidine-2,4-diones. Can. J. Chem. 2002, 80, 1302–1307. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, C.; Wang, W.-M.; Xu, L.-W.; Yang, K.-W.; Oelschlaeger, P.; He, Y. Rhodanine as potent scaffold for the development of broad-spectrum metallo-β-lactamase inhibitors. ACS Med. Chem. Lett. 2018, 9, 359–364. [Google Scholar] [CrossRef]
- Camero, D.M.; Grinalds, N.J.; Kornman, C.T.; Barba, S.; Li, L.; Weldeab, A.O.; Castellano, R.K.; Xue, J. Thin-film morphology and optical properties of photoisomerizable dono-accpetor oligothiophenes. ACS Appl. Mater. Interfaces 2023, 15, 25134–25147. [Google Scholar] [CrossRef]
- Moreno, B.; Jourdain, I.; Knorr, M.; Boudriga, S.; Strohmann, C.; Schrimpf, T. Synthesis of (Z]-3-allyl-5-(4-nitrobenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one and determination of its crystal structure. Molbank 2024, 2024, M1783. [Google Scholar] [CrossRef]
- Loncaric, M.; Molnar, M. Green synthesis of 2-thioxothazolidin-4-one derivatives in deep eutectic solvents via Knoevenagel condensation. Lett. Org. Chem. 2022, 19, 890–901. [Google Scholar] [CrossRef]
- El Ajlaoui, R.; Ouafa, A.; Mojahidi, S.; El Ammari, L.; Saadi, M.; El Mostapha, R. Unexpected Synthesis of Novel 3-Allyl-5- (Arylidene)-2-Thioxo-Thiazolidin-4-Ones in Reactions of 3-Allylrhodanine with 2-Arylidene-4-Methyl-5-Oxopyrazolidinium Ylides. Synth. Commun. 2015, 45, 2035–2042. [Google Scholar] [CrossRef]
- Sriyab, S.; Jorn-Iat, K.; Prompinit, P.; Wolschann, P.; Hannongbua, S.; Suramitr, S. Photophysical properties of 1-pyrene-based derivatives for nitroaromatic explosives detection: Experimental and theoretical studies. J. Lumin. 2018, 203, 492–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).