Cyclo[Tri(thiomethyl-1,2-phenylmethylene)]
Abstract
1. Introduction
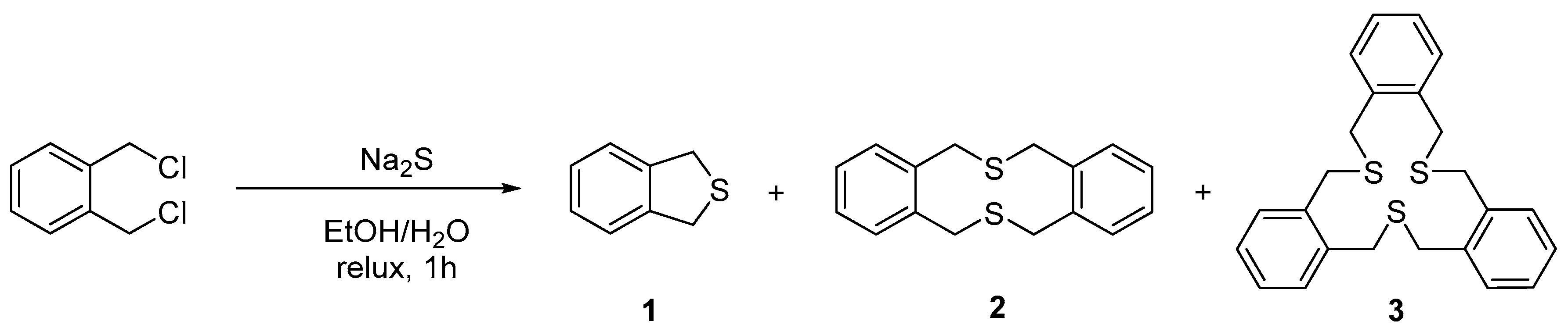
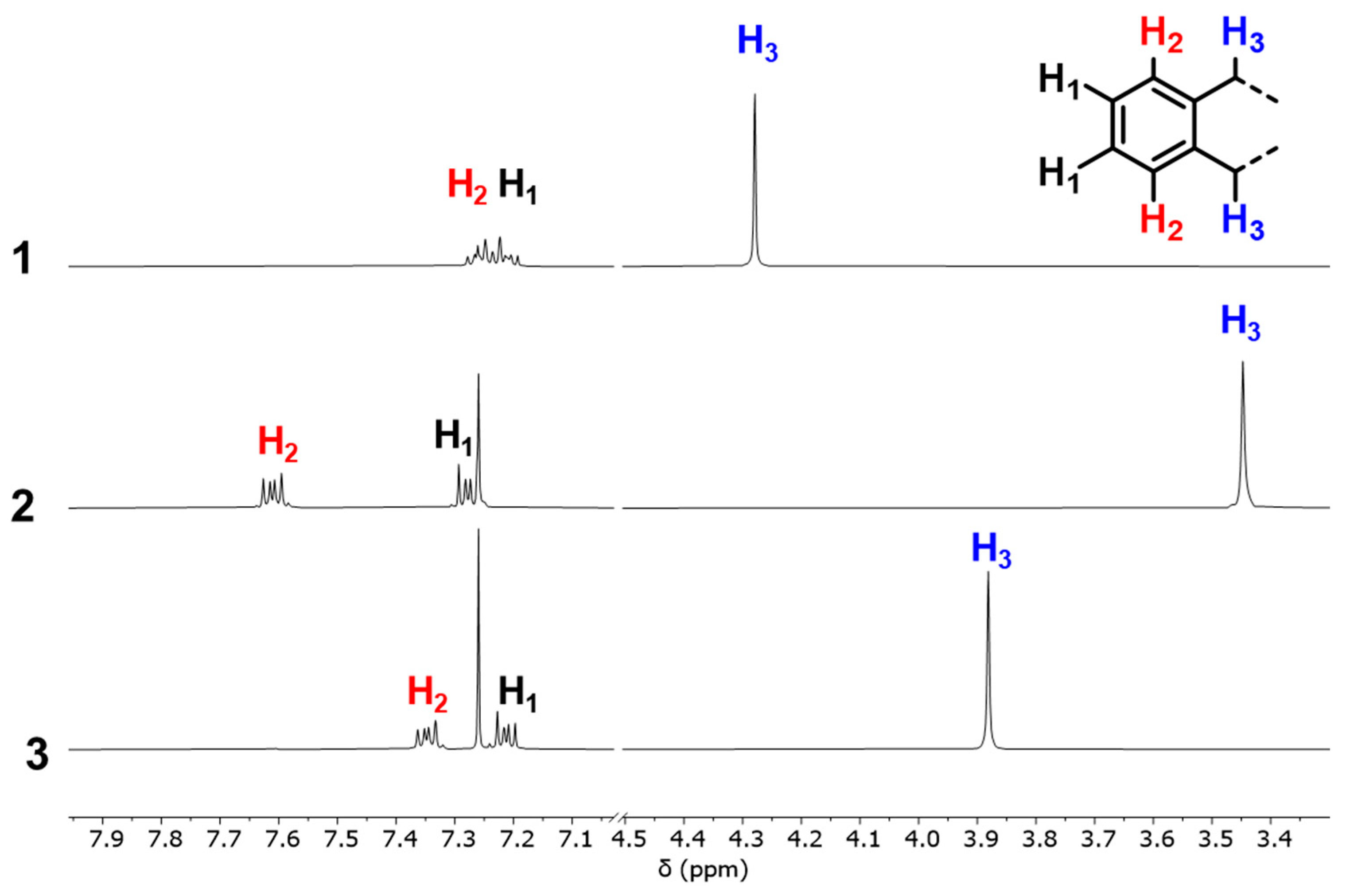
2. Results and Discussion
3. Materials and Methods
Synthesis of Cyclo[tri(thiomethyl-1,2-phenylmethylene)] (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsault, E.; Peterson, M.L. Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.K. Macrocycles: Lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015, 6, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; MacGowana, S.A.; O’Brien, J.M. Conformational control of cofactors in nature—The influence of protein-induced macrocycle distortion on the biological function of tetrapyrroles. Chem. Commun. 2015, 51, 17031–17063. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Charles, J. Pedersen: Innovator in macrocyclic chemistry and co-recipient of the 1987 Nobel Prize in chemistry. Chem. Soc. Rev. 2007, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.; Chen, C.; Eyring, E.M.; Petrucci, S. Mechanism of complexation of crown ethers as a function of alkali ions and the rigidity of the ligands. J. Phys. Chem. 1985, 89, 1357–1366. [Google Scholar] [CrossRef]
- Oral, I.; Tamm, S.; Herrmann, C.; Abetz, V. Lithium selectivity of crown ethers: The effect of heteroatoms and cavity size. Sep. Purif. Technol. 2022, 294, 121142. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Escobar, E.C.; Han, W.J.; Min, S.H.; Yook, H.; Parohinog, K.J.; Koo, S.; Kim, H.; Nisola, G.M.; Chung, W.-J. Multidentate thia-crown ethers as hyper-crosslinked macroporous adsorbent resins for the efficient Pd/Pt recovery and separation from highly acidic spent automotive catalyst leachate. Chem. Eng. J. 2021, 424, 130379. [Google Scholar] [CrossRef]
- Schneider, T.; Brüssow, N.; Yuvanc, A.; Budisa, N. Synthesis of New Aza- and Thia-Crown Ether Based Amino Acids. ChemistrySelect 2020, 5, 2854–2857. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wong, S.-Y.; Chang, D. H-Y. Synthesis of Dithie[3.3]biphenyleno(2,2′)(1,2)-,(1,3)-,(1,4)cyclophanes and Their Atropisomerism and Dynamic Stereochemistry. Tetrahedron 1993, 49, 669–676. [Google Scholar] [CrossRef]
- Grütze, J.; Vögtle, F. Synthese vielgliedriger Kohlenwasserstoffringe durch mehrfache Ringkontraktion via Sulfonpyrolyse. Chem. Ber. 1977, 110, 1978–1993. [Google Scholar] [CrossRef]
- Vögtle, F.; Ley, F. Steuerung der Bildungsselektivitat vielgliedriger Oligomerer durch Templat- und Caesium-Effekt. Chem. Ber. 1983, 116, 3000–3002. [Google Scholar] [CrossRef]
- Collins, M.S.; Carnes, M.E.; Nell, B.P.; Zakharov, L.N.; Johnson, D.W. A facile route to old and new cyclophanes via self-assembly and capture. Nat. Commun. 2016, 7, 11052. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Kerr, J.C.H.; Higgins, S.J.; Hamnett, A. A Combined Ellipsometric and In Situ Infrared (SNIFTIRS) Study of Poly(benzo-[c]-thiophene) Films. Faraday Discuss. Chem. Soc. 1989, 88, 261–275. [Google Scholar] [CrossRef]
- Kreber, R.P.; Kalischko, J. Benzo[c] thiophene mit symmetrischer Struktur: Modifizierte und optimierte Herstellung nach der S-Oxid-Route. Chem. Ber. 1991, 124, 645–654. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón Marqués, P.; Bréfuel, N.; Kammerer, C. Cyclo[Tri(thiomethyl-1,2-phenylmethylene)]. Molbank 2024, 2024, M1921. https://doi.org/10.3390/M1921
Simón Marqués P, Bréfuel N, Kammerer C. Cyclo[Tri(thiomethyl-1,2-phenylmethylene)]. Molbank. 2024; 2024(4):M1921. https://doi.org/10.3390/M1921
Chicago/Turabian StyleSimón Marqués, Pablo, Nicolas Bréfuel, and Claire Kammerer. 2024. "Cyclo[Tri(thiomethyl-1,2-phenylmethylene)]" Molbank 2024, no. 4: M1921. https://doi.org/10.3390/M1921
APA StyleSimón Marqués, P., Bréfuel, N., & Kammerer, C. (2024). Cyclo[Tri(thiomethyl-1,2-phenylmethylene)]. Molbank, 2024(4), M1921. https://doi.org/10.3390/M1921





