An Inverted-Sandwich Dichromium(I) Complex Stabilized by Guanidinate Ligands
Abstract
1. Introduction
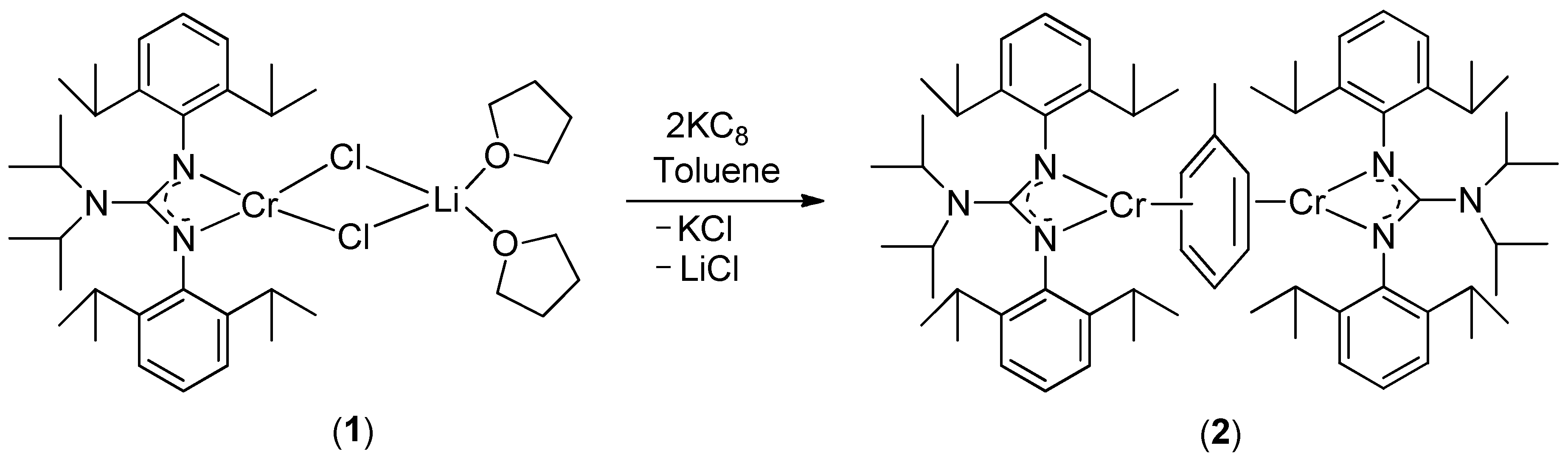
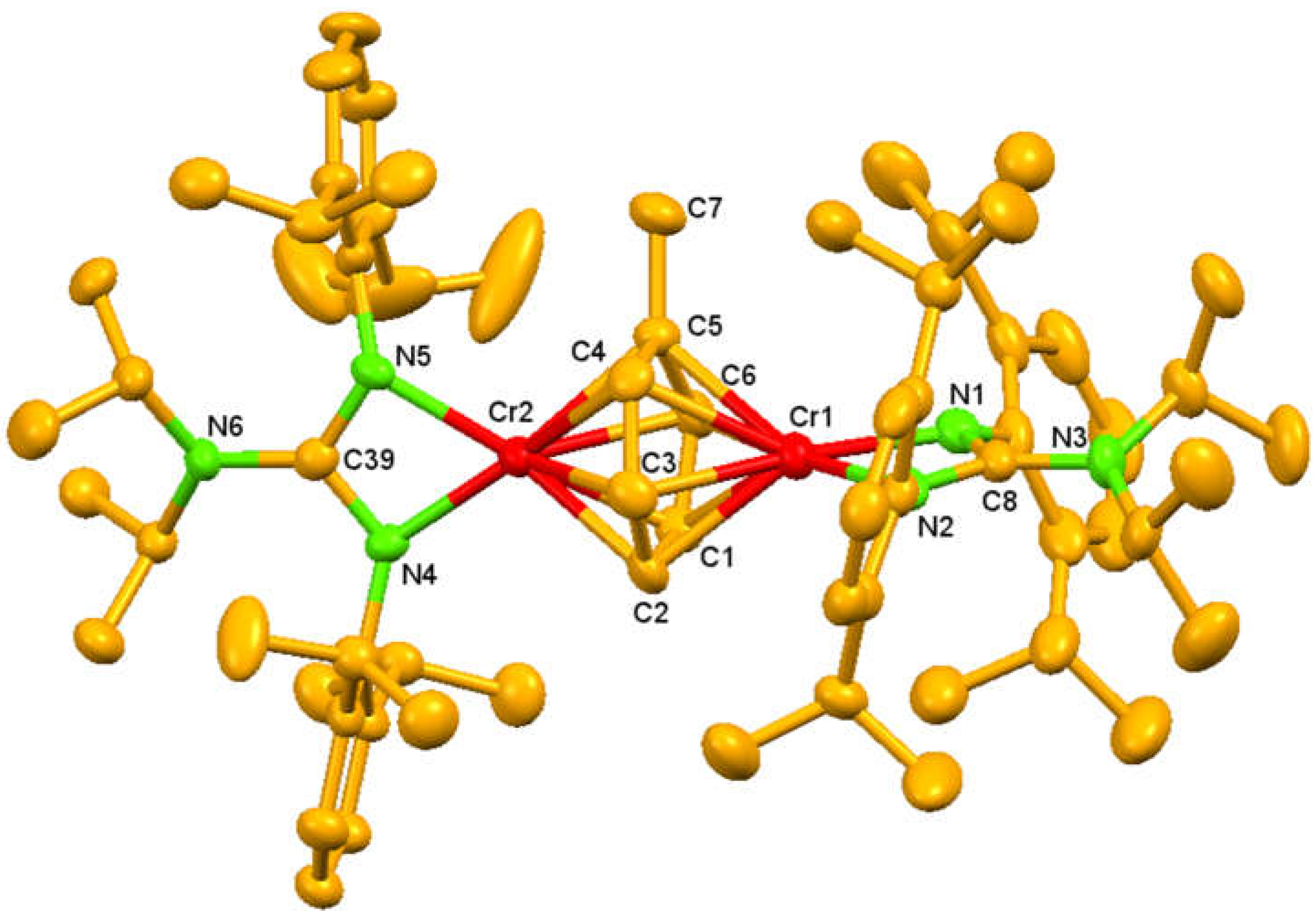
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noor, A. Recent developments in two coordinate transition metal chemistry. Coord. Chem. Rev. 2023, 476, 214941. [Google Scholar] [CrossRef]
- Wagner, F.R.; Noor, A.; Kempe, R. Ultrashort metal–metal distances and extreme bond orders. Nat. Chem. 2009, 1, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Bulky guanidinates for the stabilization of low oxidation state metallacycles. Coord. Chem. Rev. 2010, 254, 1273–1289. [Google Scholar] [CrossRef]
- Edelmann, F.T.; Hill, A.F.; Fink, M.J. Chapter Two—Recent Progress in the Chemistry of Metal Amidinates and Guanidinates: Syntheses, Catalysis and Materials. Adv. Organomet. Chem. 2013, 61, 55–374. [Google Scholar] [CrossRef]
- Edelmann, F.T. Lanthanide amidinates and guanidinates in catalysis and materials science: A continuing success story. Chem. Soc. Rev. 2012, 41, 7657–7672. [Google Scholar] [CrossRef] [PubMed]
- Fohlmeister, L.; Liu, S.; Schulten, C.; Moubaraki, B.; Stasch, A.; Cashion, J.D.; Murray, K.S.; Gagliardi, L.; Jones, C. Low-Coordinate Iron(I) and Manganese(I) Dimers: Kinetic Stabilization of an Exceptionally Short Fe-Fe Multiple Bond. Angew. Chem. Int. Ed. 2012, 51, 8294–8298. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Bauer, T.; Todorova, T.K.; Weber, B.; Gagliardi, L.; Kempe, R. The ligand-based quintuple bond-shortening concept and some of its limitations. Chem. Eur. J. 2013, 19, 9825–9832. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Glatz, G.; Müller, R.; Kaupp, M.; Demeshko, S.; Kempe, R. Metal–metal distances at the limit: Cr–Cr 1.73 Å—The importance of the ligand and its fine tuning. Z. Anorg. Allg. Chem. 2009, 635, 119–1152. [Google Scholar] [CrossRef]
- Huang, Y.S.; Huang, G.T.; Liu, Y.L.; Yu, J.S.K.; Tsai, Y.C. Reversible Cleavage/Formation of the Chromium–Chromium Quintuple Bond in the Highly Regioselective Alkyne Cyclotrimerization. Angew. Chem. Int. Ed. 2017, 56, 15427–15431. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Wang, P.Y.; Chen, S.A.; Chen, J.M. Inverted-Sandwich Dichromium(I) Complexes Supported by Two β-Diketiminates: A Multielectron Reductant and Syntheses of Chromium Dioxo and Imido. J. Am. Chem. Soc. 2007, 129, 8066–8067. [Google Scholar] [CrossRef]
- Monillas, W.H.; Yap, G.P.A.; Theopold, K.H. A Tale of Two Isomers: A Stable Phenyl Hydride and a High-Spin (S = 3) Benzene Complex of Chromium. Angew. Chem. Int. Ed. 2007, 46, 6692–6694. [Google Scholar] [CrossRef] [PubMed]
- Bénard, M.; Rohmer, M.M.; López, X.; Theopold, K.H. A Tale of Two Isomers (Continued): Is the Phenyl Hydride Complex of Chromium More Stable than Its Benzene-Bridged Isomer? Angew. Chem. Int. Ed. 2008, 47, 5597–5599. [Google Scholar] [CrossRef] [PubMed]
- Plíva, J.; Johns, J.W.C.; Goodman, L. Infrared bands of isotopic benzenes: ν13 and ν14 of 13C6D6. J. Mol. Spectrosc. 1990, 140, 214–225. [Google Scholar] [CrossRef]
- Madelung, O. (Ed.) Landolt-Bornstein, Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin, Germany, 1987; Volume 15. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR 97: A new tool for crystal determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2018; Program for Crystal Structure Refinement Universität Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Farrugia, L.J. WinGX Suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
| Empirical Formula | C76H112Cr2N6 |
|---|---|
| Formula weight | 1213.71 |
| crystal system | monoclinic |
| space group | P21/c |
| a [Å] | 15.108(3) |
| b [Å] | 29.155(6) |
| c [Å] | 13.9971(8) |
| α [deg] | 90 |
| β [deg] | 101.19(3) |
| γ [deg] | 90 |
| V, [Å3] | 7555(3) |
| Z | 4 |
| crystal size, [mm3] | 0.33 × 0.28 × 0.09 |
| ρcalcd, [g cm−3] | 1.067 |
| µ, [mm−1] (Mo Kα) | 0.329 |
| T, [K] | 133(2) |
| 2θ range, [deg] | 2.74–49.39 |
| no. of reflections unique | 14,233 |
| no. of reflections obs. [I > 2σ (I)] | 5576 |
| no. of parameters | 739 |
| wR2 (all data) | 0.1280 |
| R value [I > 2σ (I)] | 0.0683 |
| Goodness of fit | 0.786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.; Qayyum, S.; Dickert, A.; El Oirdi, M. An Inverted-Sandwich Dichromium(I) Complex Stabilized by Guanidinate Ligands. Molbank 2024, 2024, M1901. https://doi.org/10.3390/M1901
Noor A, Qayyum S, Dickert A, El Oirdi M. An Inverted-Sandwich Dichromium(I) Complex Stabilized by Guanidinate Ligands. Molbank. 2024; 2024(4):M1901. https://doi.org/10.3390/M1901
Chicago/Turabian StyleNoor, Awal, Sadaf Qayyum, Andre Dickert, and Mohamed El Oirdi. 2024. "An Inverted-Sandwich Dichromium(I) Complex Stabilized by Guanidinate Ligands" Molbank 2024, no. 4: M1901. https://doi.org/10.3390/M1901
APA StyleNoor, A., Qayyum, S., Dickert, A., & El Oirdi, M. (2024). An Inverted-Sandwich Dichromium(I) Complex Stabilized by Guanidinate Ligands. Molbank, 2024(4), M1901. https://doi.org/10.3390/M1901








