Synthesis of Novel Thiazoles Based on (+)-Usnic Acid
Abstract
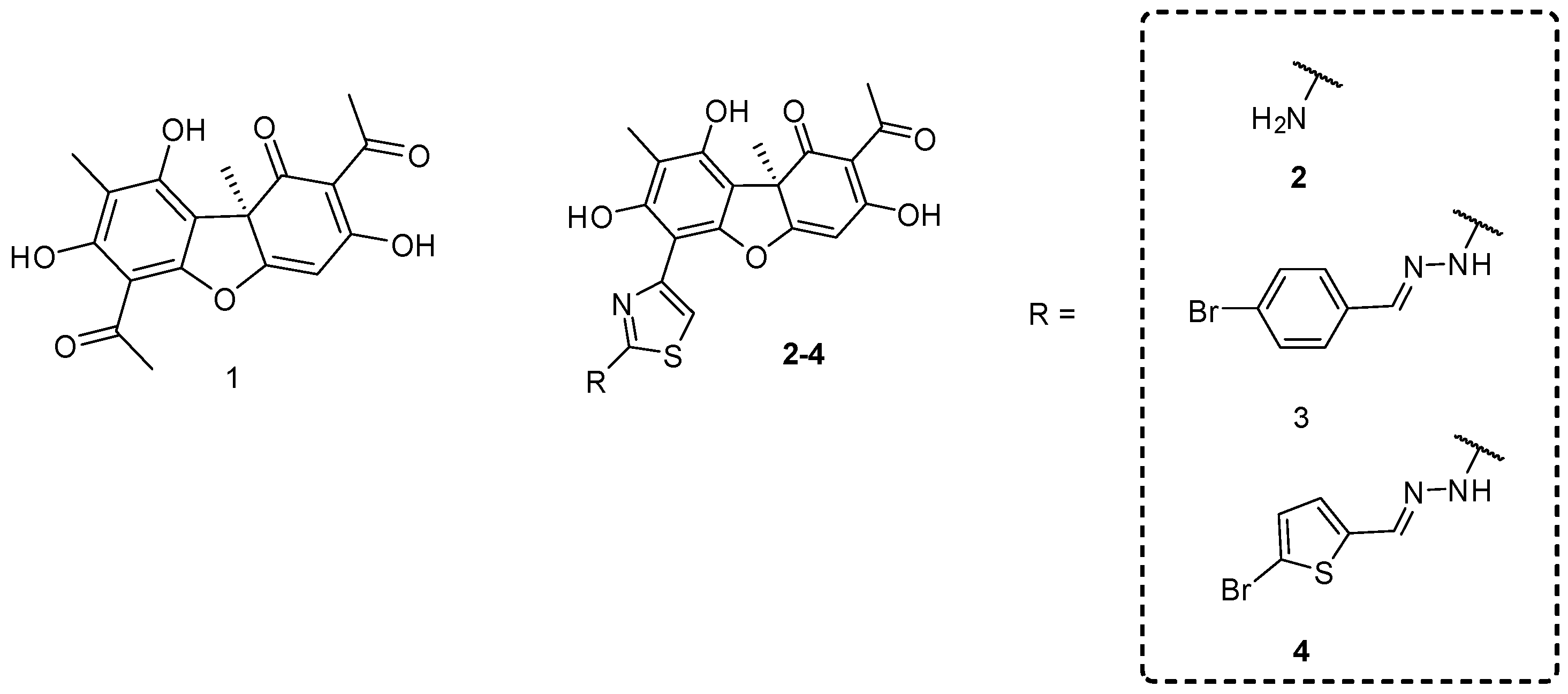
1. Introduction
2. Results and Discussion
3. Materials and Methods
The Synthesis of Compounds 5a–e
- (A)
- A total of 1 mmol of carboxylic acid was placed in a flask with a solution of oxalyl chloride in methylene chloride (2 mmol in 2 mL). A few drops of DMF were added to the mixture and left to be stirred at room temperature for one hour. Afterwards, the solvent was removed using a rotary evaporator. The resulting mixture was dissolved in 10 mL of acetone, and the solution was heated to 50 °C. Sodium thiocyanate (1 mmol) was added to the solution. After 15 min, the solution was cooled, and the precipitate that formed (sodium chloride) was filtered off. The resulting solution was evaporated on a rotary evaporator. An aqueous solution of ammonia (120 µL, 30% in water) was added to the resulting mixture and left to be stirred for 30 min. The precipitate that formed was filtered off, washed with water, and dried in air.
- (B)
- A total of 1 mmol of bromousnic acid 7 was dissolved in 35 mL of methanol. The corresponding acylthiourea 8a–e (1 mmol) was added to the solution. The solution was stirred for 1 h. Then, the solution was diluted with water, and the precipitate that formed was filtered, washed with water and dried in air.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luzina, O.A.; Salakhutdinov, N.F. Usnic acid and its derivatives for pharmaceutical use: A patent review (2000–2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Bekker, O.B.; Sokolov, D.N.; Luzina, O.A.; Komarova, N.I.; Gatilov, Y.V.; Andreevskaya, S.N.; Smirnova, T.G.; Maslov, D.A.; Chernousova, L.N.; Salakhutdinov, N.F.; et al. Synthesis and activity of (+)-usnic acid and (-)-usnic acid derivatives containing 1,3-thiazole cycle against Mycobacterium tuberculosis. Med. Chem. Res. 2015, 24, 2926–2938. [Google Scholar] [CrossRef]
- Yarovaya, O.I.; Filimonov, A.S.; Baev, D.S.; Borisevich, S.S.; Chirkova, V.Y.; Zaykovskaya, A.V.; Mordvinova, E.D.; Belenkaya, S.V.; Shcherbakov, D.N.; Luzina, O.A.; et al. Usnic acid based thiazole-hydrazones as multi-targeting inhibitors of a wide spectrum of SARS-CoV-2 viruses. New J. Chem. 2023, 47, 19865–19879. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.E.A. Ionization Constants and Ionization Profiles. Compr. Med. Chem. II 2007, 5, 357–397. [Google Scholar] [CrossRef]
- Siddiqui, N.; Alam, M.; Ahsan, W. Synthesis, anticonvulsant and toxicity evaluation of 2-(1H-indol-3-yl) acetyl-N-(substituted phenyl) hydrazine carbothioamides and their related heterocyclic derivatives. Acta Pharm. 2008, 58, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hegab, M.I.; Abdel-Fattah, A.S.; Yousef, N.M.; Nour, H.F.; Mostafa, A.M.; Ellithey, M. Synthesis, X-ray Structure, and Pharmacological Activity of Some 6, 6-Disubstituted Chromeno [4,3-b]- and Chromeno-[3, 4-c]- quinolines. Arch. Pharm. 2007, 340, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sana, A.; Khan, S.W.; Zaidi, J.H.; Ambreen, N.; Khan, K.M.; Perveen, S. Syntheses and antimicrobial activities of amide derivatives of 4-[(2-isopropyl-5-methylcyclohexyl) oxo]-4-oxobutanoic acid. Nat. Sci. 2011, 3, 855–861. [Google Scholar] [CrossRef]
- Graybill, T.L.; Ross, M.J.; Gauvin, B.R.; Gregory, J.S.; Harris, A.L.; Ator, M.A.; Rinker, J.M.; Dolle, R.E. Synthesis and evaluation of azapeptide-derived inhibitors of serine and cysteine proteases. Bioorg. Med. Chem. Lett. 1992, 2, 1375–1380. [Google Scholar] [CrossRef]
- Bi, Y.; Xu, J.; Sun, F.; Wu, X.; Ye, W.; Sun, Y.; Huang, W. Synthesis and biological activity of 28-amide derivatives of 23-hydroxy betulinicacid as antitumor agent candidates. Med. Chem. 2013, 9, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Midura-Nowaczek, K.R.; Lepietuszko, I.; Bruzgo, I.; Markowska, A. Biological activity of amide derivatives of lysine. Acta Pol. Pharm. 2008, 65, 377–381. [Google Scholar] [PubMed]
- Hu, L.H.; Chen, Z.L.; Xie, Y.Y. Synthesis and biological activity of amide derivatives of ginkgolide A. J. Asian Nat. Prod. Res. 2001, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Jawaid, T.; Dar, U.A.; Shah, S.A. Amide as a Potential Pharmacophore for Drug Designing of Novel Anticonvulsant Compounds. In Chemistry of Biologically Potent Natural Products and Synthetic Compounds; Wiley-Scrivener Publishing: Beverly, MA, USA, 2021; pp. 319–342. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonov, A.S.; Luzina, O.A.; Salakhutdinov, N.F. Synthesis of Novel Thiazoles Based on (+)-Usnic Acid. Molbank 2024, 2024, M1894. https://doi.org/10.3390/M1894
Filimonov AS, Luzina OA, Salakhutdinov NF. Synthesis of Novel Thiazoles Based on (+)-Usnic Acid. Molbank. 2024; 2024(4):M1894. https://doi.org/10.3390/M1894
Chicago/Turabian StyleFilimonov, Aleksandr S., Olga A. Luzina, and Nariman F. Salakhutdinov. 2024. "Synthesis of Novel Thiazoles Based on (+)-Usnic Acid" Molbank 2024, no. 4: M1894. https://doi.org/10.3390/M1894
APA StyleFilimonov, A. S., Luzina, O. A., & Salakhutdinov, N. F. (2024). Synthesis of Novel Thiazoles Based on (+)-Usnic Acid. Molbank, 2024(4), M1894. https://doi.org/10.3390/M1894





