[(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine
Abstract
1. Introduction
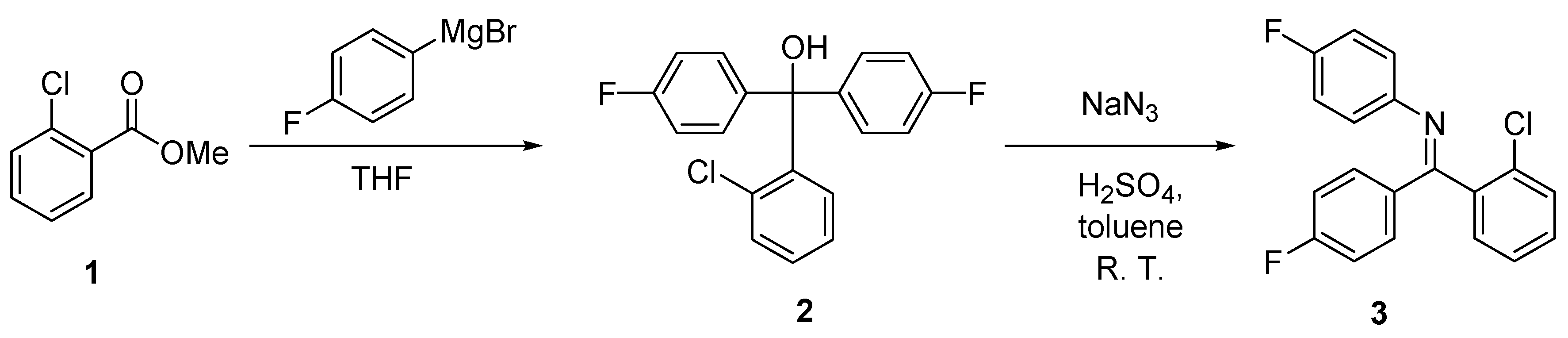
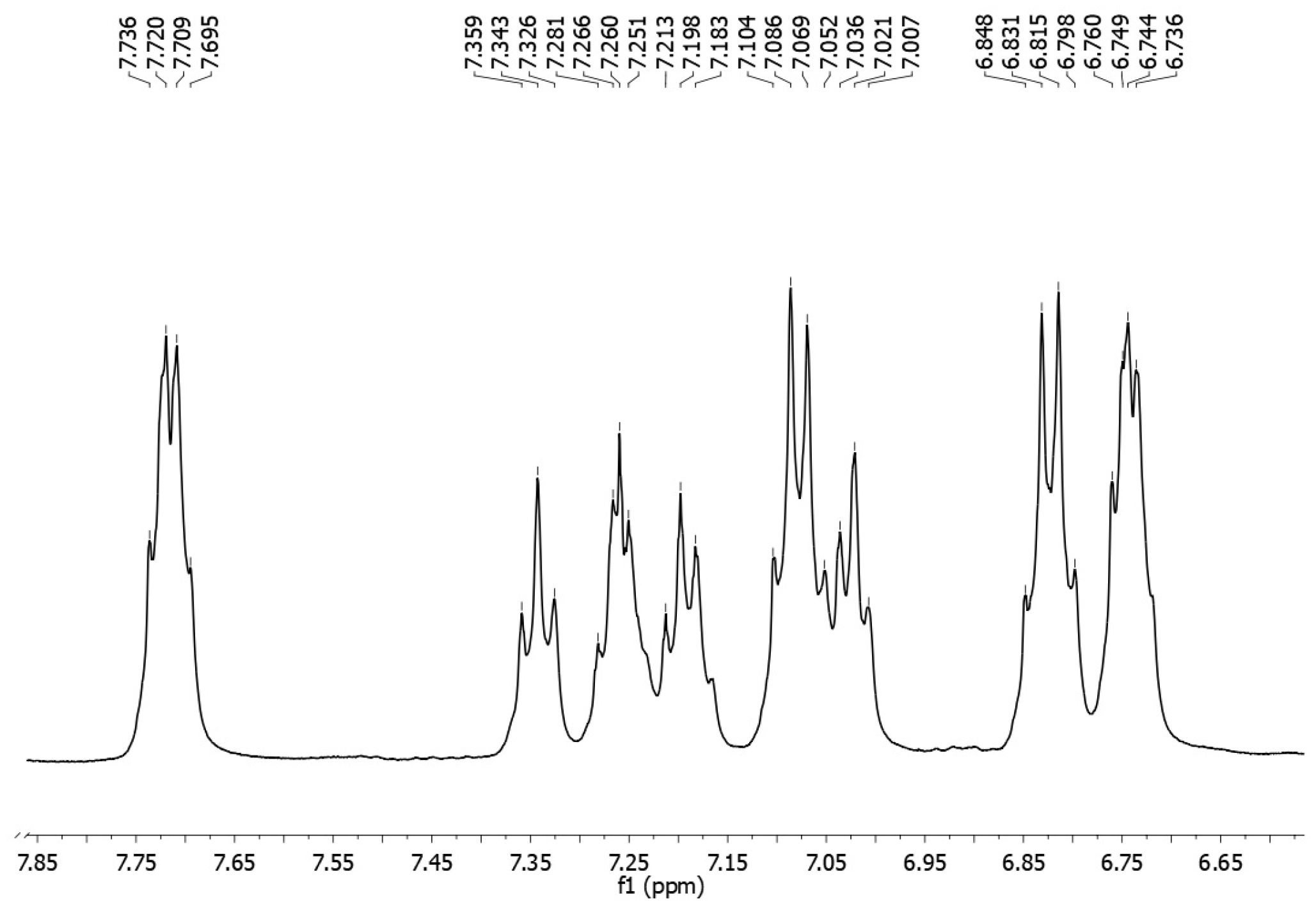
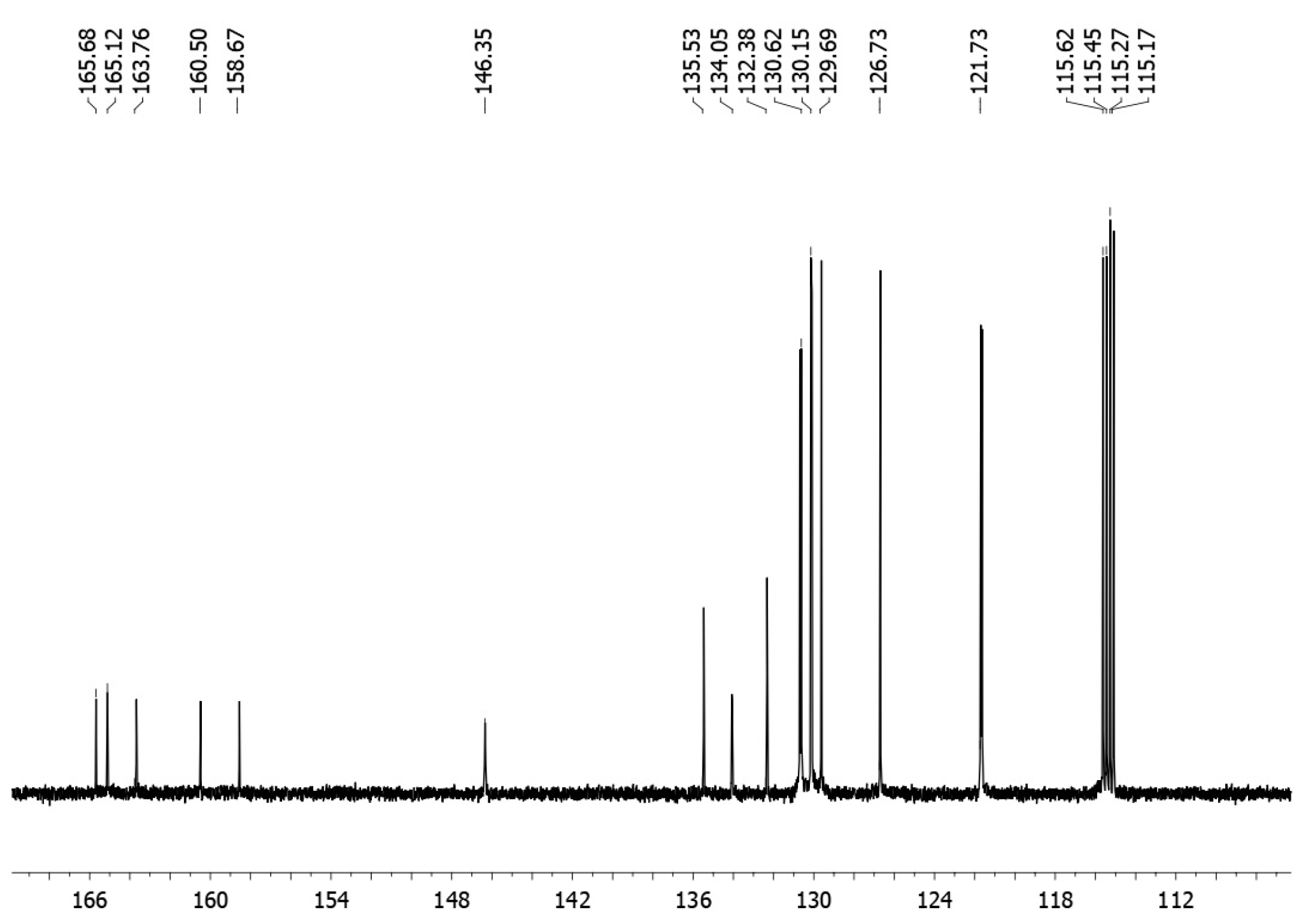
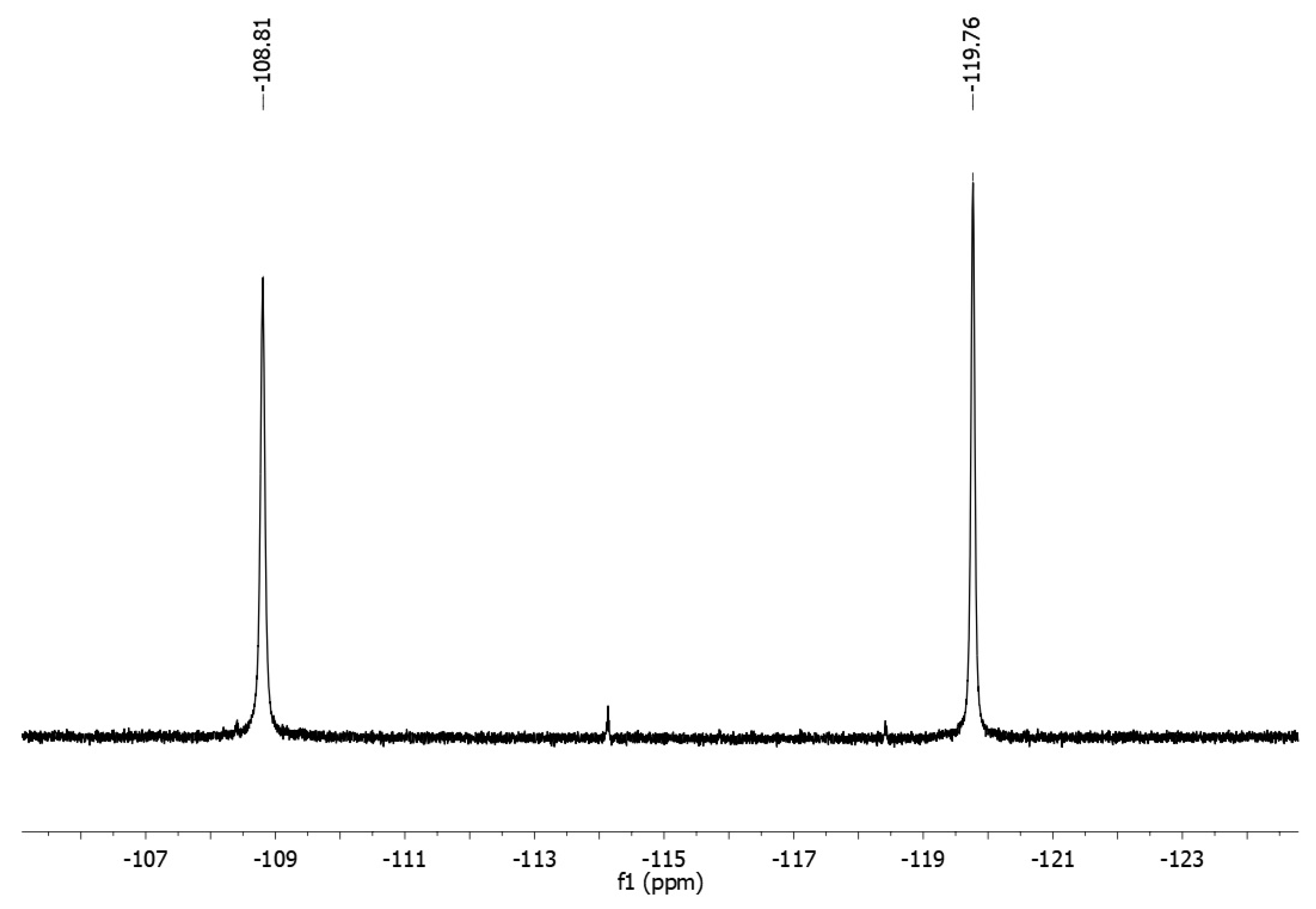
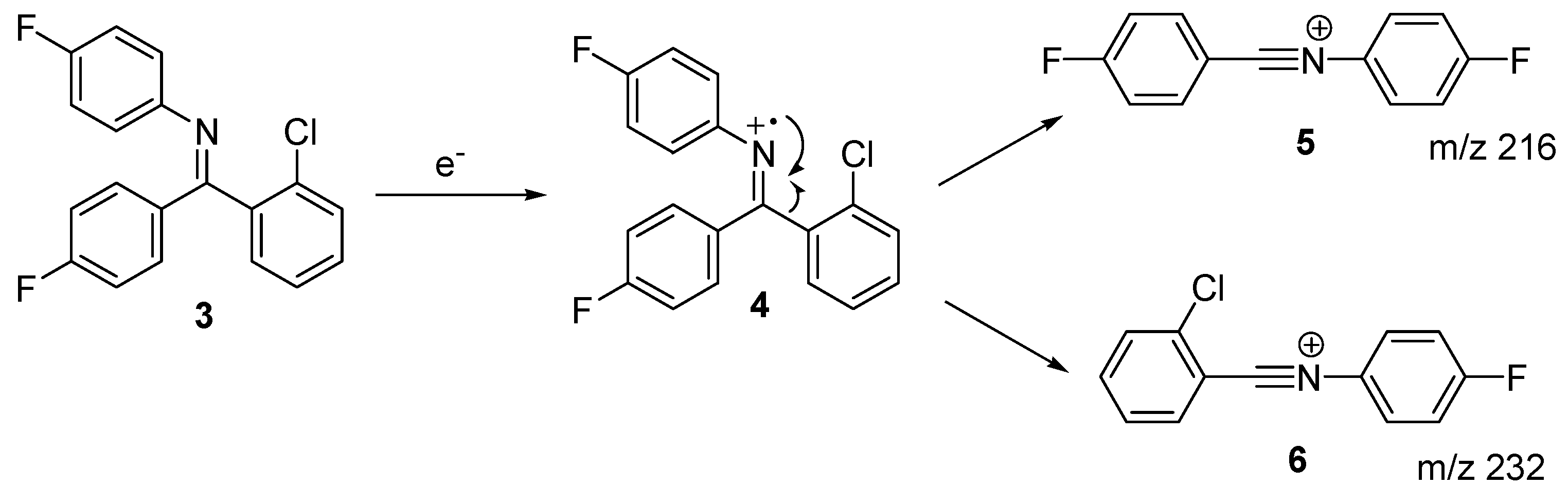
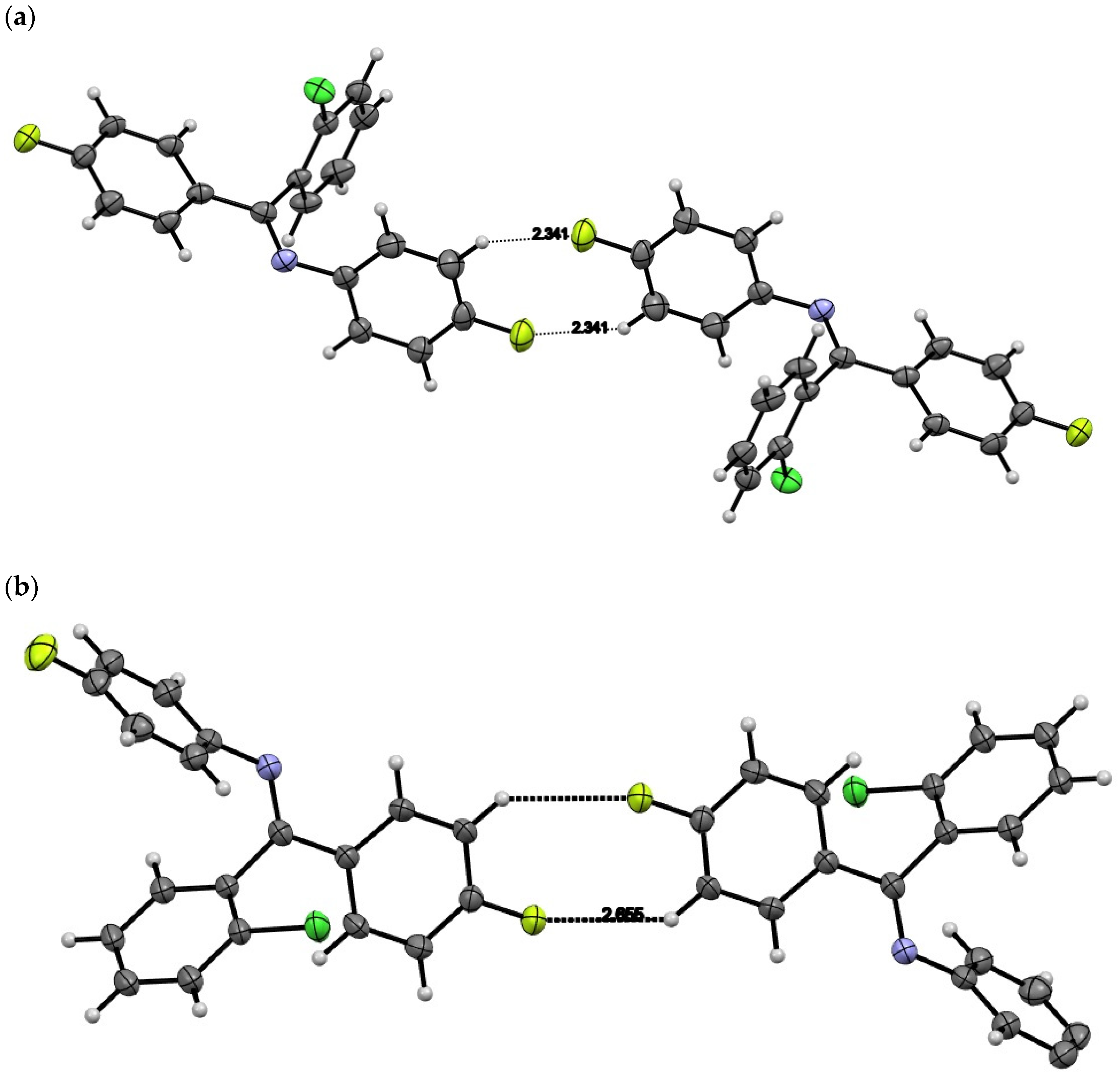
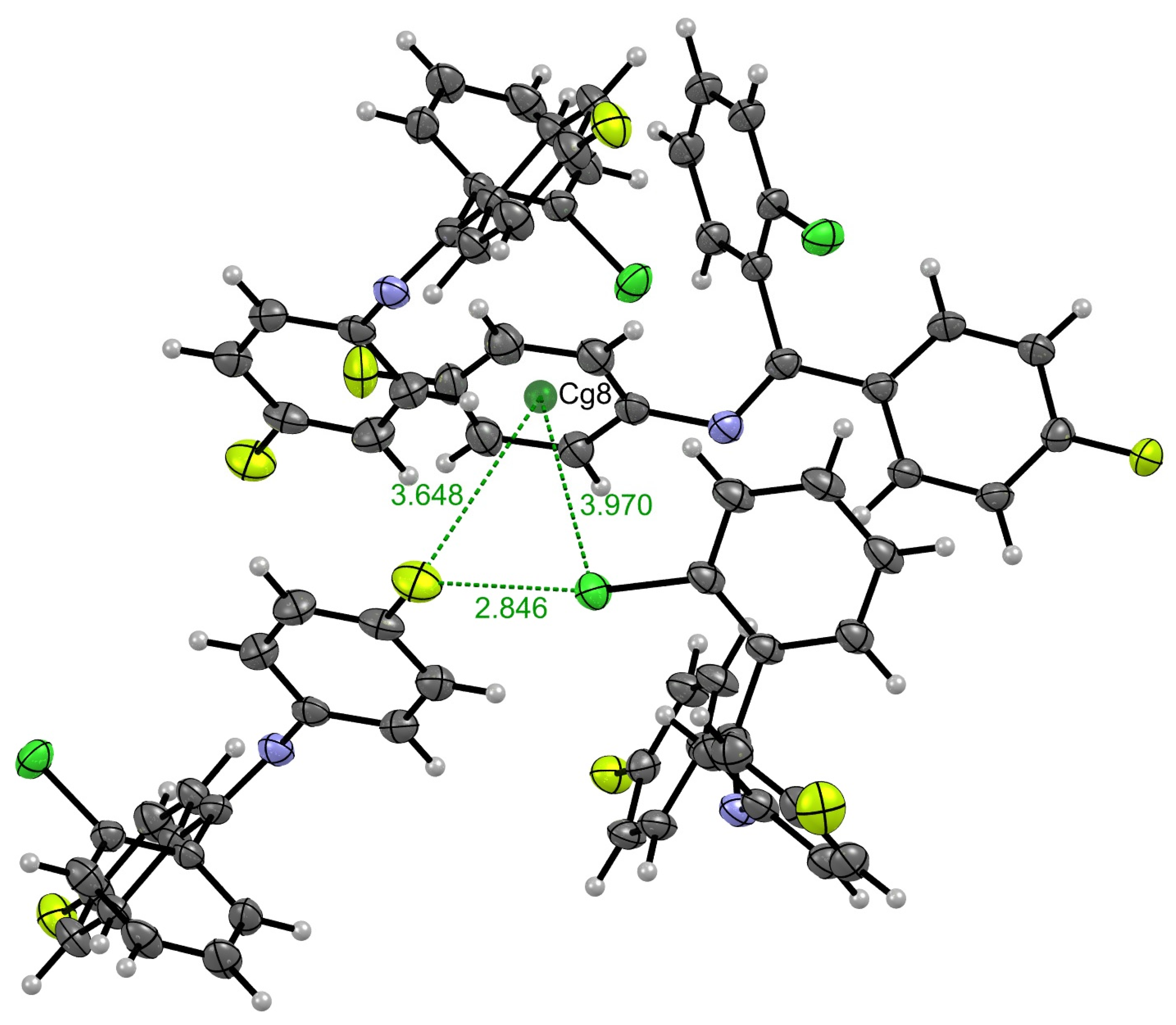
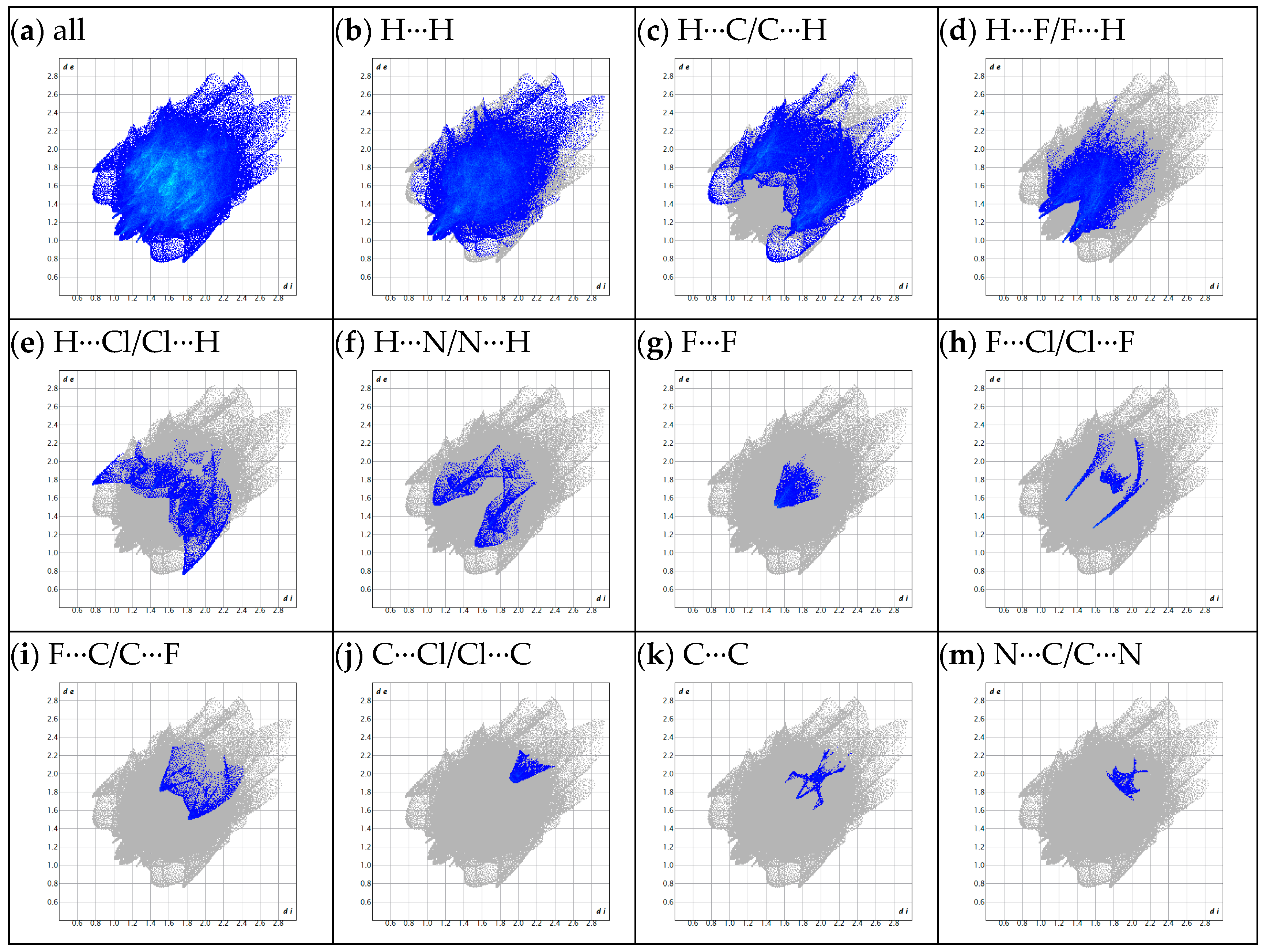
2. Results and Discussion
3. Materials and Methods
Synthesis of [(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrobleski, A.; Coombs, T.C.; Huh, C.W.; Sze-Wan Li, S.W.; Aubé, J. The Schmidt Reaction. Org. React. Chem. 2021, 71, 1–320. [Google Scholar]
- Looker, J.J. Thermal Decomposition of Some 5- Substituted 5-Azido-5H-dibenzo[u,d]cycloheptenes. A Transannular Nitrene Addition. J. Org. Chem. 1971, 36, 1045–1047. [Google Scholar] [CrossRef]
- Astier, A.; Plat, M.M. Synthesis of natural products via tertiary azides 2-alkyl and cis 2,6 alkylpiperidine alkaloids. Tetrahedron Lett. 1978, 19, 2051–2052. [Google Scholar] [CrossRef]
- Pearson, W.H.; Fang, W.K. Synthesis of Benzo-Fused 1-Azabicyclo[m.n.0]alkanes via the Schmidt Reaction: A Formal Synthesis of Gephyrotoxin. J. Org. Chem. 2000, 65, 7158–7174. [Google Scholar]
- Pearson, W. Aliphatic Azides as Lewis Bases. Application to the Synthesis of Heterocyclic Compounds. J. Heterocycl. Chem. 1996, 33, 1489–1495. [Google Scholar] [CrossRef]
- Gnagi, L.; Arnold, R.; Giornal, F.; Jangra, H.; Kapat, A.; Erich Nyfeler, E.; Scharer, R.; Zipse, H.; Renaud, P. Stereoselective and Stereospecific Triflate-Mediated Intramolecular Schmidt Reaction: Ready Access to Alkaloid Skeletons. Angew. Chem. Int. Ed. 2021, 60, 10179–10185. [Google Scholar] [CrossRef]
- Zong-Quan Liao, Z.Q.; Dong, C.; Carlson, K.E.; Srinivasan, S.; Nwachukwu, J.C.; Chesnut, R.W.; Sharma, A.; Nettles, K.W.; Katzenellenbogen, J.A.; Zhou, H.B. Triaryl-Substituted Schiff Bases Are High-Affinity Subtype-Selective Ligands for the Estrogen Receptor. J. Med. Chem. 2014, 57, 3532–3545. [Google Scholar] [CrossRef] [PubMed]
- Shtaiwi, A.; Adnan, R.; Khairuddean, M.; Khan, S.U. Computational investigations of the binding mechanism of novel benzophenone imine inhibitors for the treatment of breast cancer. RSC Adv. 2019, 9, 35401–35416. [Google Scholar] [CrossRef]
- Roy, H.N.; Pitchaiah, A.; Kim, M.; Hwanga, I.T.; Lee, K.I. Protective group-free synthesis of new chiral diamines via direct azidation of 1,1-diaryl-2-aminoethanols. RSC Adv. 2013, 3, 3526–3530. [Google Scholar] [CrossRef]
- Yue, Y.; Li, J.; Xie, X.; Guo, C.; Yin, X.; Yin, Q.; Chen, Y.; Pana, Y.; Ding, C. Ortho-hydroxyl effect and proton transfer via ion–neutral complex: The fragmentation study of protonated imine resveratrol analogues in mass spectrometry. J. Mass Spectrom. 2016, 51, 518–523. [Google Scholar] [CrossRef]
- Tucker, P.A.; Hoekstra, A.; ten Cate, J.M.; Vos, A. The Crystal and Molecular Structure of N-(Diphenylmethylene)aniline at –160 °C. Act. Cryst. 1975, B31, 733–737. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Baydere, C.; Tasci, M.; Dege, N.; Arslan, M.; b Yusuf Atalay, Y.; Golenya, I.A. Crystal structure and Hirshfeld surface analysis of (E)-2-(2,4,6-trimethylbenzylidene)-3,4-dihydronaphthalen-1(2H)-one. Acta Cryst. E 2019, E75, 746–750. [Google Scholar] [CrossRef]
- Carter, D.J.; Raiteri, P.; Barnard, K.R.; Gielink, R.; Mocerino, M.; Skelton, B.W.; Vaughan, J.G.; Ogden, M.I.; Rohl, A.L. Difference Hirshfeld fingerprint plots: A tool for studying polymorphs. CrystEngComm 2017, 19, 2201–2215. [Google Scholar] [CrossRef]
- Cedillo-Cruz, A.; Martínez-Otero, D.; Barroso-Flores, J.; Cuevas-Yañez, E. α-(1,2,3-Triazolyl)-acetophenone: Synthesis and theoretical studies of crystal and 2,4-dinitrophenylhydrazine cocrystal structures. J. Mol. Struct. 2022, 1264, 133225. [Google Scholar] [CrossRef]
- Shikhaliyev, N.Q.; Turktekin Celikesir, S.T.; Akkurt, M.; Bagirova, K.B.; Suleymanova, G.T.; Toze, F.A.A. Crystal structure and Hirshfeld surface analysis of (E)-1-(4-chlorophenyl)-2-[2,2-dichloro-1-(4-fluorophenyl)ethenyl]diazene. Acta Cryst. E 2019, E75, 465–469. [Google Scholar] [CrossRef]
- Chauhan, D.P.; Varma, S.J.; Vijeta, A.; Banerjee, P.; Talukdar, P. A 1,3-amino group migration route to form acrylamidines. Chem. Commun. 2014, 50, 323–325. [Google Scholar] [CrossRef]
- APEX, Bruker. SAINT, and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; Mccabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem. Phys. 1993, 98, 1358–1737. [Google Scholar] [CrossRef]
- Hassner, A.; Stern, M.; Gottlieb, H.E.; Frolow, F. Utility of a Polymeric Azide Reagent in the Formation of Di- and Triazidomethane. Their NMR Spectra and the X-ray Structure of Derived Triazoles. J. Org. Chem. 1990, 55, 2304–2306. [Google Scholar] [CrossRef]
- Hassner, A.; Stern, M. Synthesis of Alkyl Azides with a Polymeric Reagent. Angew. Chem. Int. Ed. 1986, 25, 478–479. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]






| Crystal Data | 3 |
|---|---|
| Empirical formula | C19H12ClF2N |
| Formula weight | 327.75 |
| Temperature (K) | 100(2) |
| Radiation type | Mo Kα |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions (Å, °) | |
| a | 14.9045(11) |
| b | 18.2310(14) |
| c | 11.9976(9) |
| α | 90 |
| β | 108.2613(13) |
| γ | 90 |
| Volume (Å3) | 3095.9(4) |
| Z | 8 |
| Density (calculated, Mg/m3) | 1.406 |
| Absorption coefficient μ (mm−1) | 0.265 |
| F(000) | 1344 |
| Crystal size (mm3) | 0.571 × 0.496 × 0.390 |
| Θ range (deg) | 2.108 to 27.445 |
| Index ranges | −19 ≤ h ≤ 19, −23 ≤ k ≤ 23, −15 ≤ l ≤ 15 |
| Reflections collected | 31,893 |
| Independent reflections | 5902 [R(int) = 0.0365] |
| Data/restraints/parameters | 7074/1336/607 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0554, wR2 = 0.1081 |
| R indices (all data) | R1 = 0.0444, wR2 = 0.1021 |
| Largest diff. peak and hole (e Å−3) | 0.461, −0.361 |
| Bond | Distance (Å) | Bond | Angle (°) |
|---|---|---|---|
| N(1)-C(1) | 1.276(2) | N(1)-C(1)-C(8) | 118.42(15) |
| N(1)-C(14) | 1.423(2) | N(1)-C(1)-C(2) | 122.22(18) |
| C(3)-Cl(1) | 1.737(2) | C(8)-C(1)-C(2) | 119.19(17) |
| F(1)-C(11) | 1.3645(19) | C(1)-N(1)-C(14) | 121.11(15) |
| F(2)-C(17) | 1.360(2) | F(1)-C11(3)-C(10) | 118.69(15) |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | ∠D–H···A | Symmetry Operation |
|---|---|---|---|---|---|
| C29–H29···F4 | 0.95 | 2.34 | 3.190(2) | 148.6 | −x, −y, −z + 1 |
| C32–H32···F1 | 0.95 | 2.57 | 3.403(2) | 145.8 | x, −y − 1/2, z − 1/2 |
| C35A–H35A···F3A | 0.95 | 2.43 | 3.320(13) | 155 | −x + 1, −y + 1, −z + 1 |
| C31–H31···Cg7 | 0.95 | 2.84 | 3.7134(3) | 153 | −x + 1, −y, −z + 1 |
| C24A–H24A···Cg9 | 0.95 | 2.9 | 3.7947(3) | 157 | x − 1, −y − 1/2, z − 3/2 |
| C3–Cl1···Cg8 | 1.737(2) | 3.9700(3) | 4.7062(4) | 104.14(1) | x, −y − 1/2, z − 3/2 |
| C30–F4···Cg8 | 1.362(2) | 3.6476(3) | 4.1689(3) | 102.92(1) | −x + 1, y − 1/2, −z + 3/2 |
| Cg(I)–Cg(J) | Cg–Cg (Å) a | Symmetry Operation |
|---|---|---|
| Cg3···Cg7 | 4.1009(3) | x, −y − 1/2, z−1/2 |
| Cg5···Cg7 | 4.2046(3) | x, −y − 1/2, z−1/2 |
| Cg6···Cg8 | 4.3525(3) | x, −y − 1/2, z−1/2 |
| Cg1···Cg2 | 4.3802(3) | x, −y − 1/2, z−1/2 |
| Cg2···Cg4 | 4.5044(3) | x, −y − 1/2, z−1/2 |
| Cg8···Cg9 | 4.5669(4) | x, −y − 1/2, z−1/2 |
| Cg2i···Cg5ii | 4.9103(4) | x, −y − 1/2, z−3/2 |
| Cg2i···Cg4ii | 4.9214(4) | x, −y − 1/2, z−3/2 |
 | Ring | Dihedral Angle (°) |
| A/B | 72.97 | |
| A/C | 60.86 | |
| B/C | 73.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilchis-Valdés, S.; Cedillo-Cruz, A.; García-Eleno, M.A.; Martínez-Otero, D.; Cuevas-Yañez, E. [(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine. Molbank 2024, 2024, M1892. https://doi.org/10.3390/M1892
Vilchis-Valdés S, Cedillo-Cruz A, García-Eleno MA, Martínez-Otero D, Cuevas-Yañez E. [(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine. Molbank. 2024; 2024(4):M1892. https://doi.org/10.3390/M1892
Chicago/Turabian StyleVilchis-Valdés, Salvador, Alberto Cedillo-Cruz, Marco A. García-Eleno, Diego Martínez-Otero, and Erick Cuevas-Yañez. 2024. "[(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine" Molbank 2024, no. 4: M1892. https://doi.org/10.3390/M1892
APA StyleVilchis-Valdés, S., Cedillo-Cruz, A., García-Eleno, M. A., Martínez-Otero, D., & Cuevas-Yañez, E. (2024). [(2-Chlorophenyl)-(4-fluorophenyl)methylene]-(4-fluorophenyl)amine. Molbank, 2024(4), M1892. https://doi.org/10.3390/M1892







