Benzo[d][1,3]oxathiole-2-thione
Abstract
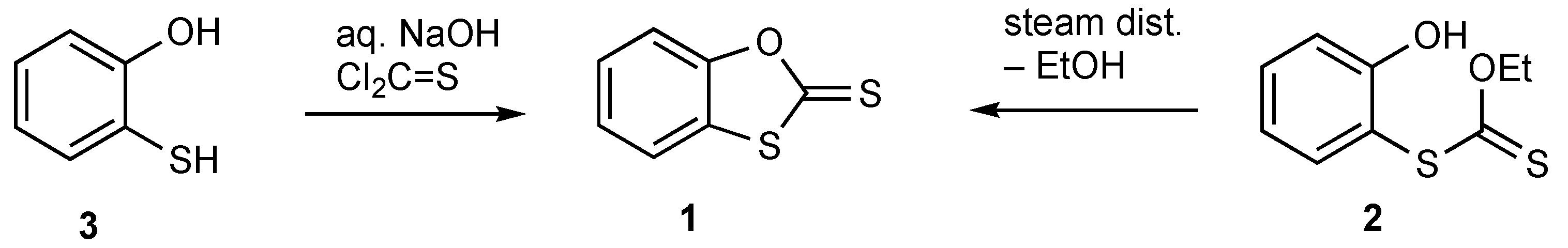
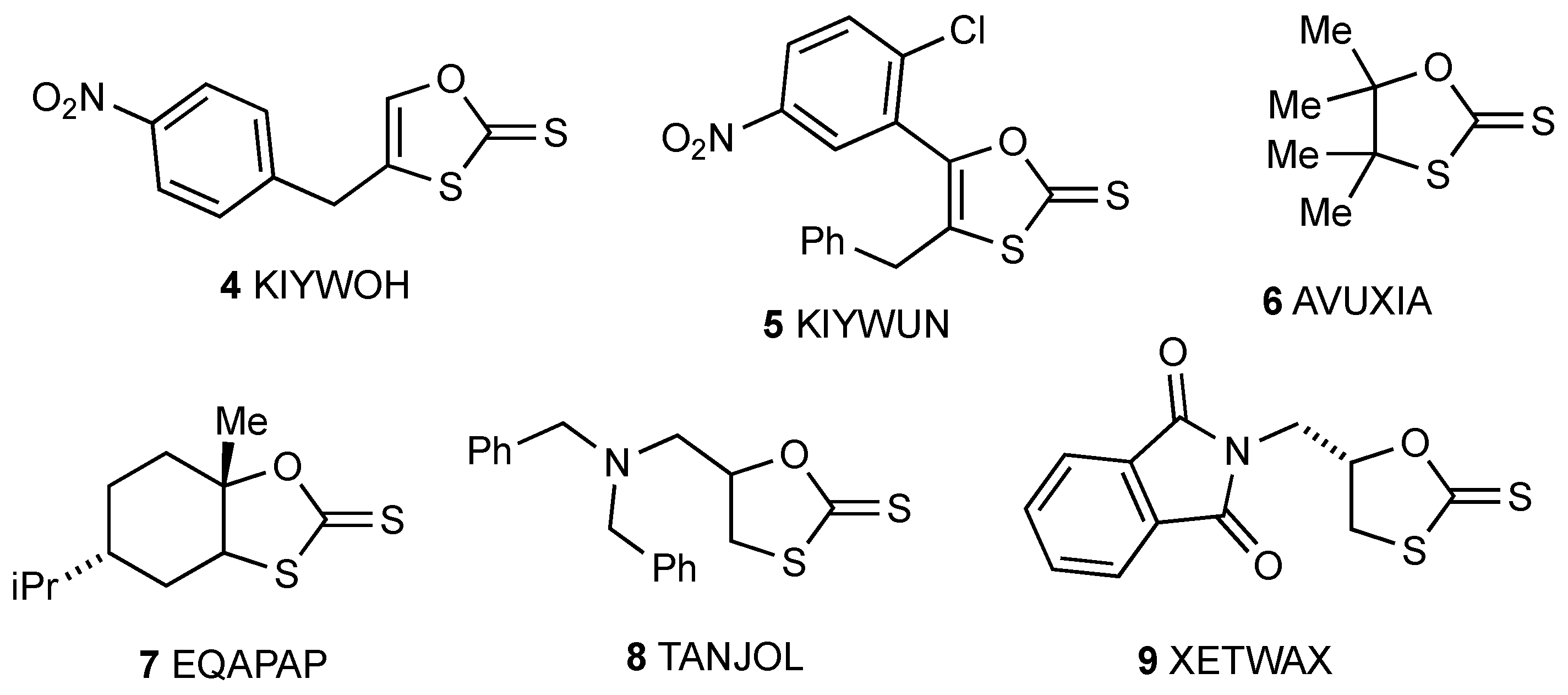
1. Introduction
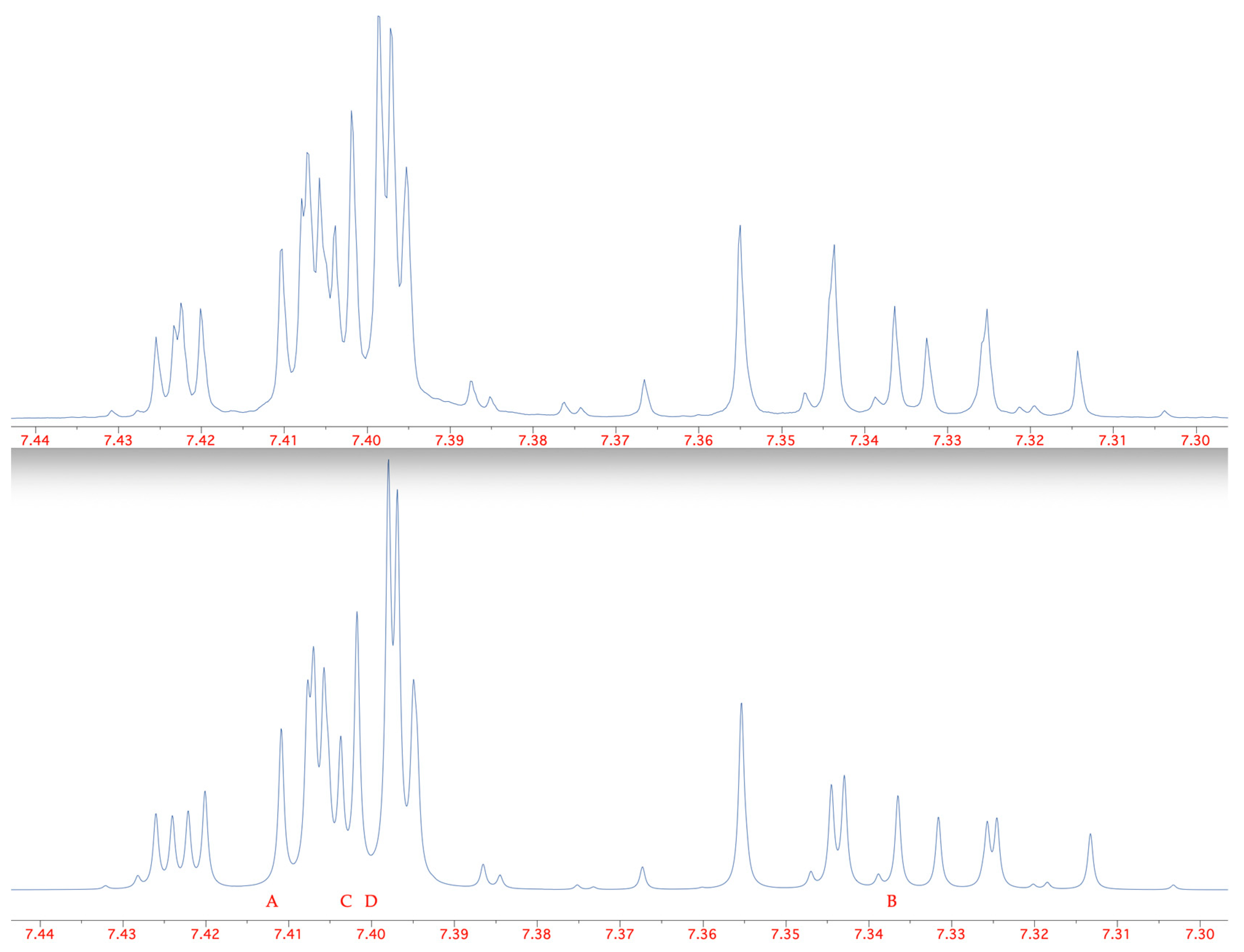
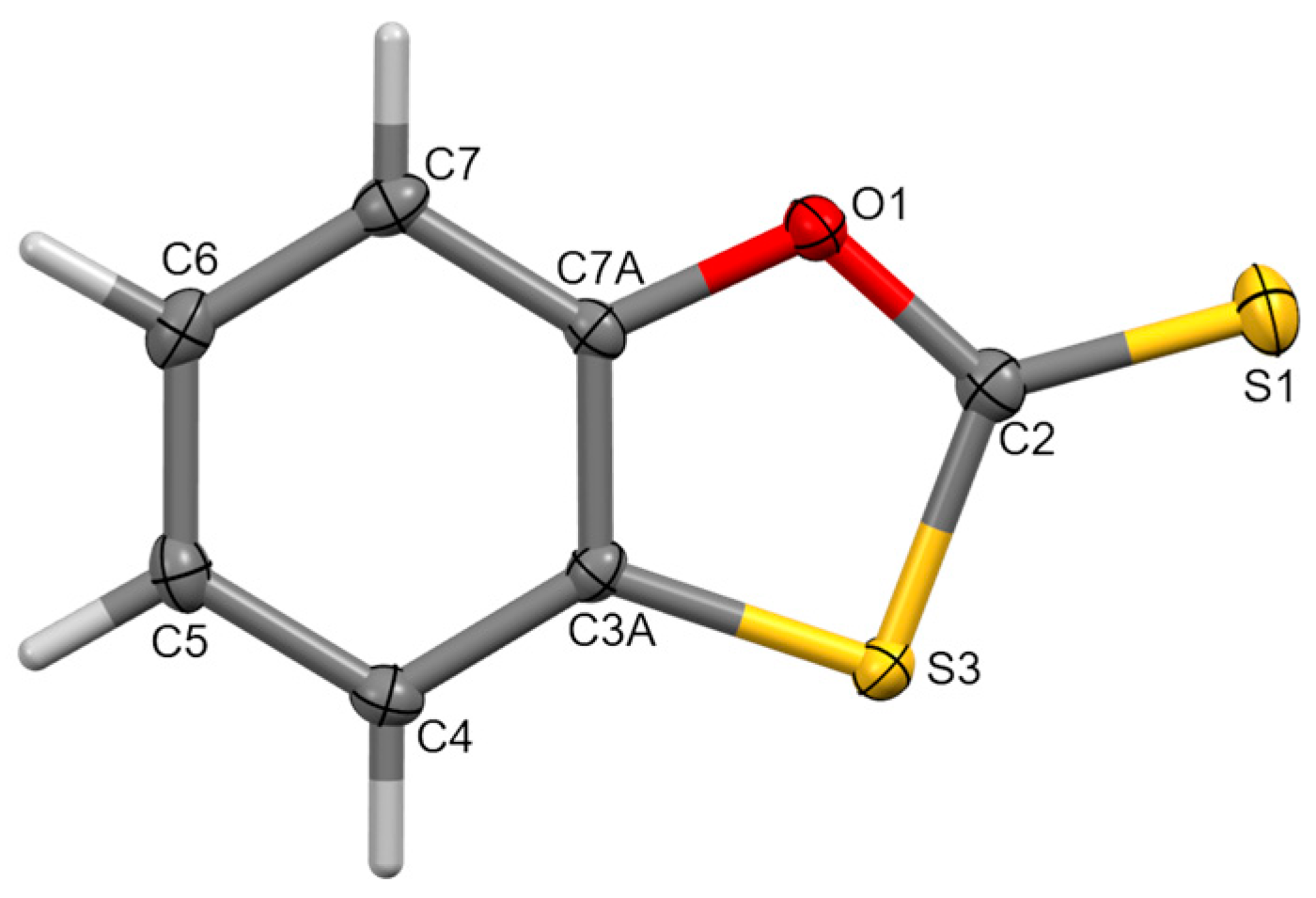
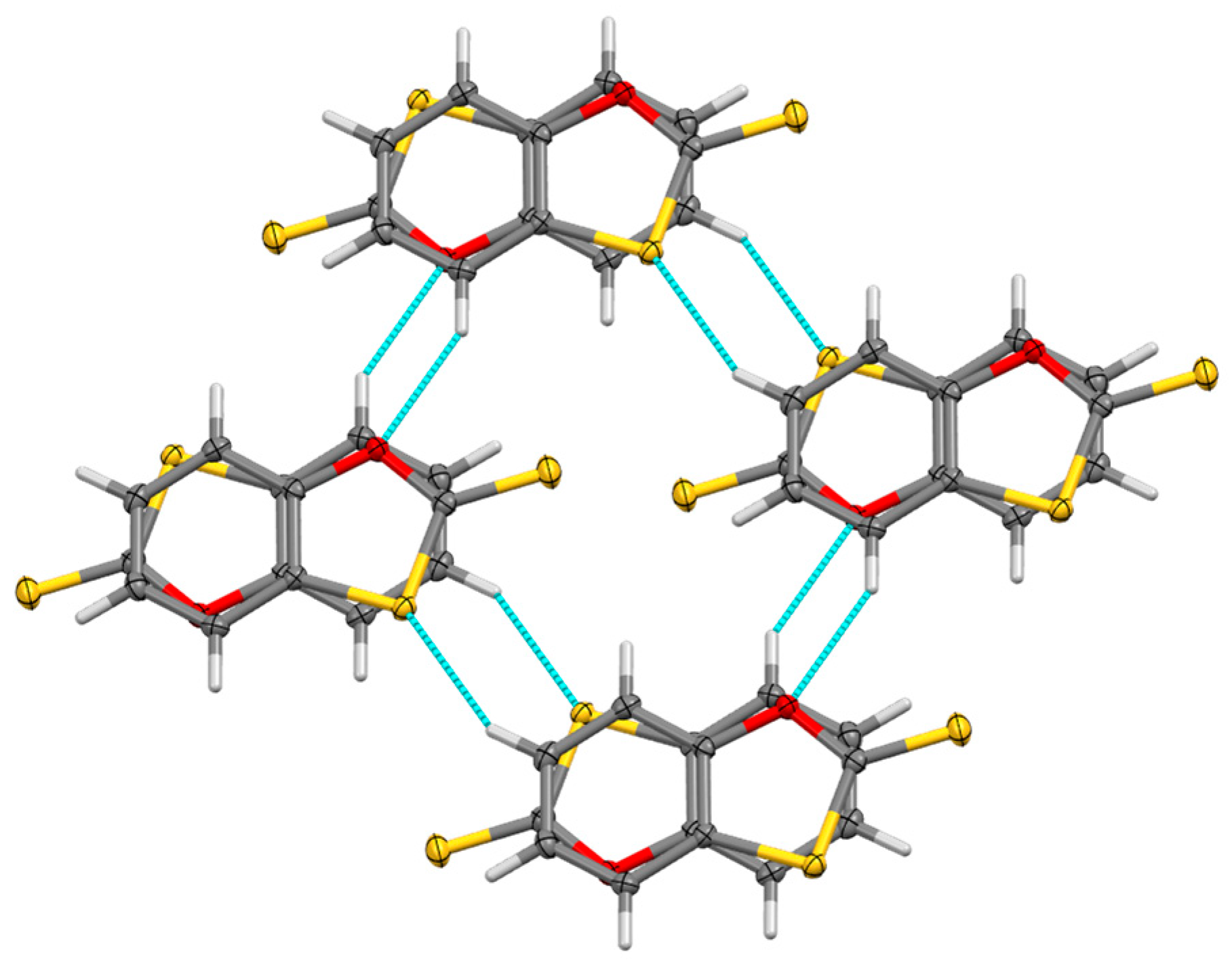
2. Results
3. Experimental
3.1. General Experimental Details
3.2. Synthesis of Benzo[d][1,3]oxathiole-2-thione 1
3.3. X-ray Structure Determination of 1
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedländer, P.; Mauthner, F. Zur Kenntnis der Schwefelfarbstoffe. Z. Farben-Text.-Ind. 1904, 3, 333–337. [Google Scholar]
- Greenwood, D.; Stevenson, H.A. Benz-1:3-oxathioles, benz-1:4-oxathien, and αω-bisarylthioalkanes. J. Chem. Soc. 1953, 1514–1519. [Google Scholar] [CrossRef]
- Copeland, C.; Stick, R.V. The reaction of N-dichloromethylene-N,N-dimethylammonium chloride (Viehe’s salt) with thiophenols and phenols: A synthesis of unsymmetrical diaryl carbonates. Aust. J. Chem. 1984, 37, 1483–1487. [Google Scholar] [CrossRef]
- Jordis, U. Hydride reduction of 1,3-benzodithiole-2-thiones and -selones. J. Chem. Res (S) 1986, 432, J. Chem. Res. (M) 1986, 3401–3413. [Google Scholar]
- Nakayama, J.; Kimata, A.; Taniguchi, H.; Takahashi, F. Reactions of benzyne with 1,3-benzodithiole-2-thione and related compounds: Formation of novel tetracyclic sulfonium salts and their reactions leading to dibenzo-1,3,6,-trithiocin derivatives. Bull. Chem. Soc. Jpn. 1996, 69, 2349–2354. [Google Scholar] [CrossRef]
- Aitken, R.A.; Cordes, D.B.; Goyal, A.; McKay, A.P. Benzo[d][1,2,3]oxadithiole 2-Oxide. Molbank 2024, 2024, M1803. [Google Scholar] [CrossRef]
- Savarimuthu, S.A.; Prakash, D.G.L.; Thomas, S.A.; Gandhi, T. DBU-Mediated intermolecular 5-exo-dig cyclization of propargyl alcohols and carbon disulfide to [1,3]-oxathiole-2-thiones. ChemistrySelect 2018, 3, 13087–13090. [Google Scholar] [CrossRef]
- Diebler, J.; Spannenberg, A.; Werner, T. Regio- and stereoselective synthesis of dithiocarbonates under ambient and solvent-free conditions. ChemCatChem 2016, 8, 2027–2030. [Google Scholar] [CrossRef]
- Diebler, J.; Spannenberg, A.; Werner, T. Atom economical synthesis of di- and trithiocarbonates by the lithium tert-butoxide catalyzed addition of carbon disulfide to epoxides and thiiranes. Org. Biomol. Chem. 2016, 14, 7480–7489. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Yoshida, Y.; Endo, T. Cationic ring-opening polymerization of a five membered cyclic dithiocarbonate having a tertiary amine moiety. Polym. Chem. 2021, 13, 267–274. [Google Scholar] [CrossRef]
- Okada, M.; Nishiyori, R.; Kaneko, S.; Igawa, K.; Shirakawa, S. KI–tetraethylene glycol complex as an effective catalyst for the synthesis of cyclic thiocarbonates from epoxides and CS2. Eur. J. Org. Chem. 2018, 2018, 2022–2027. [Google Scholar] [CrossRef]
- CrysAlisPro; v1.171.43.109a; Rigaku Oxford Diffraction, Rigaku Corporation: Tokyo, Japan, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
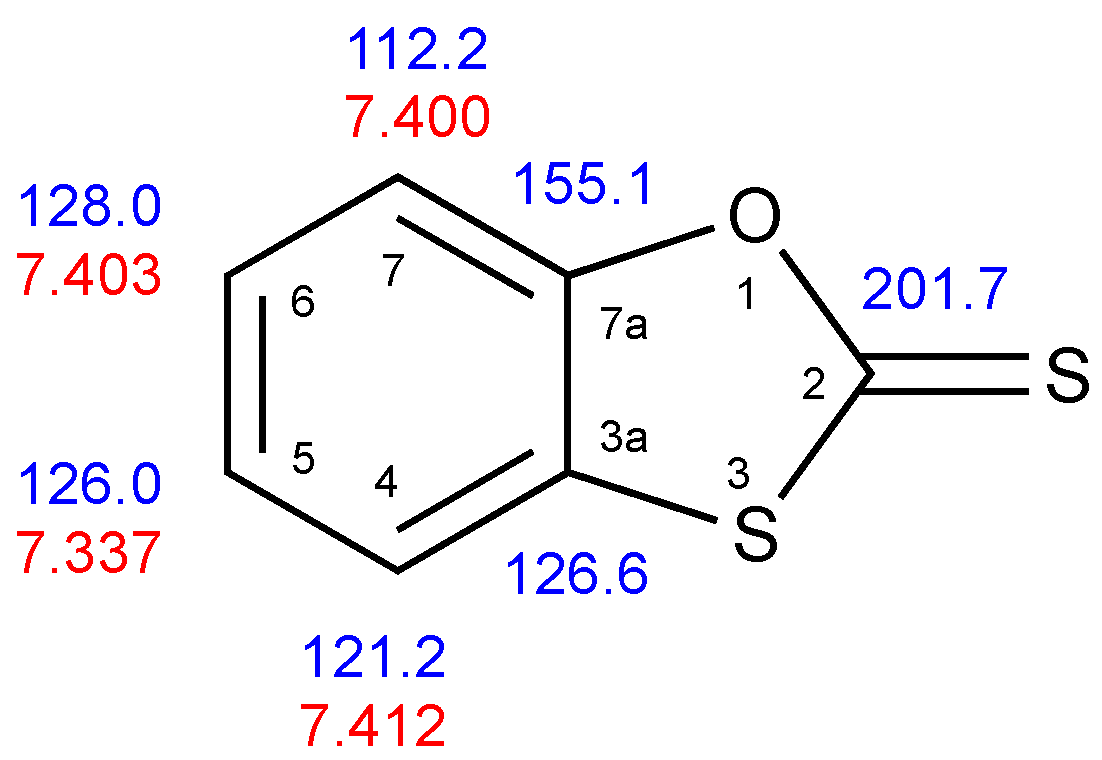
| Position | δC | δH |
|---|---|---|
| 2 | 201.7 | – |
| 3a | 126.6 | – |
| 4 | 121.2 | 7.412 |
| 5 | 126.0 | 7.337 |
| 6 | 128.0 | 7.403 |
| 7 | 112.2 | 7.400 |
| 7a | 155.1 | – |
| Compound | 3JH4–H5 | 4JH4–H6 | 5JH4–H7 | 3JH5–H6 | 4JH5–H7 | 3JH6–H7 |
|---|---|---|---|---|---|---|
| 1 | 8.0 | 1.8 | 0.3 | 7.4 | 1.7 | 8.5 |
| Bond Length | Angle | ||
|---|---|---|---|
| S(3)–C(2) | 1.7399(16) | C(3a)–S(3)–C(2) | 91.42(7) |
| C(2)–S(1) | 1.6293(16) | S(3)–C(2)–O(1) | 111.77(11) |
| C(2)–O(1) | 1.3625(18) | S(3)–C(2)–S(1) | 125.58(10) |
| O(1)–C(7a) | 1.3891(17) | S(1)–C(2)–O(1) | 122.65(12) |
| C(7a)–C(3a) | 1.393(2) | C(2)–O(1)–C(7a) | 112.89(11) |
| C(3a)–S(3) | 1.7422(15) | O(1)–C(7a)–C(3a) | 114.29(13) |
| C(7a)–C(3a)–S(3) | 109.61(11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cordes, D.B.; Cottineau, L.; McKay, A.P. Benzo[d][1,3]oxathiole-2-thione. Molbank 2024, 2024, M1891. https://doi.org/10.3390/M1891
Aitken RA, Cordes DB, Cottineau L, McKay AP. Benzo[d][1,3]oxathiole-2-thione. Molbank. 2024; 2024(4):M1891. https://doi.org/10.3390/M1891
Chicago/Turabian StyleAitken, R. Alan, David B. Cordes, Lauryne Cottineau, and Aidan P. McKay. 2024. "Benzo[d][1,3]oxathiole-2-thione" Molbank 2024, no. 4: M1891. https://doi.org/10.3390/M1891
APA StyleAitken, R. A., Cordes, D. B., Cottineau, L., & McKay, A. P. (2024). Benzo[d][1,3]oxathiole-2-thione. Molbank, 2024(4), M1891. https://doi.org/10.3390/M1891









