Methyl 6,7-Difluoro-2-[(4-fluorobenzyl)sulfanyl]-4-hydroxyquinoline-3-carboxylate
Abstract
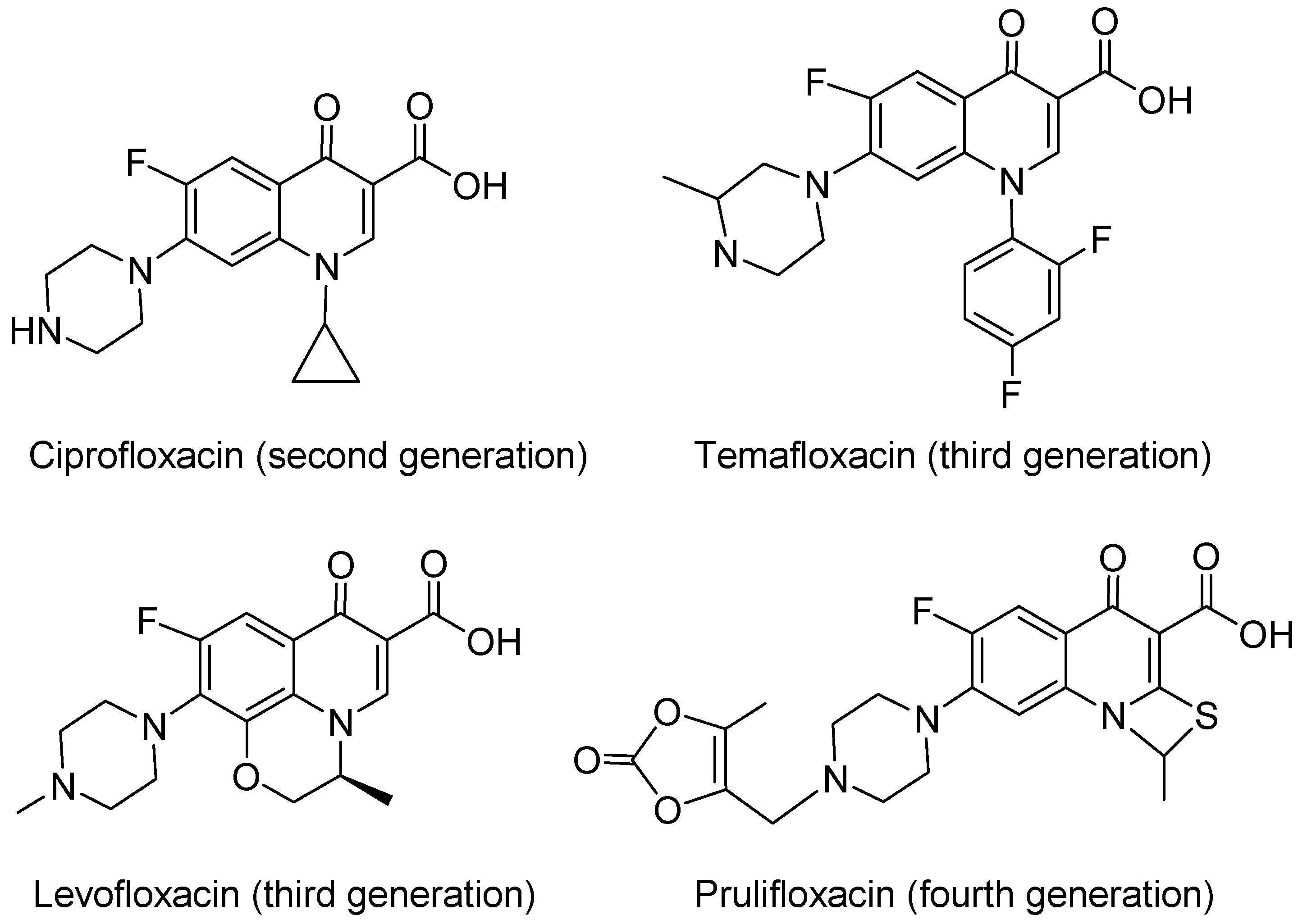
1. Introduction
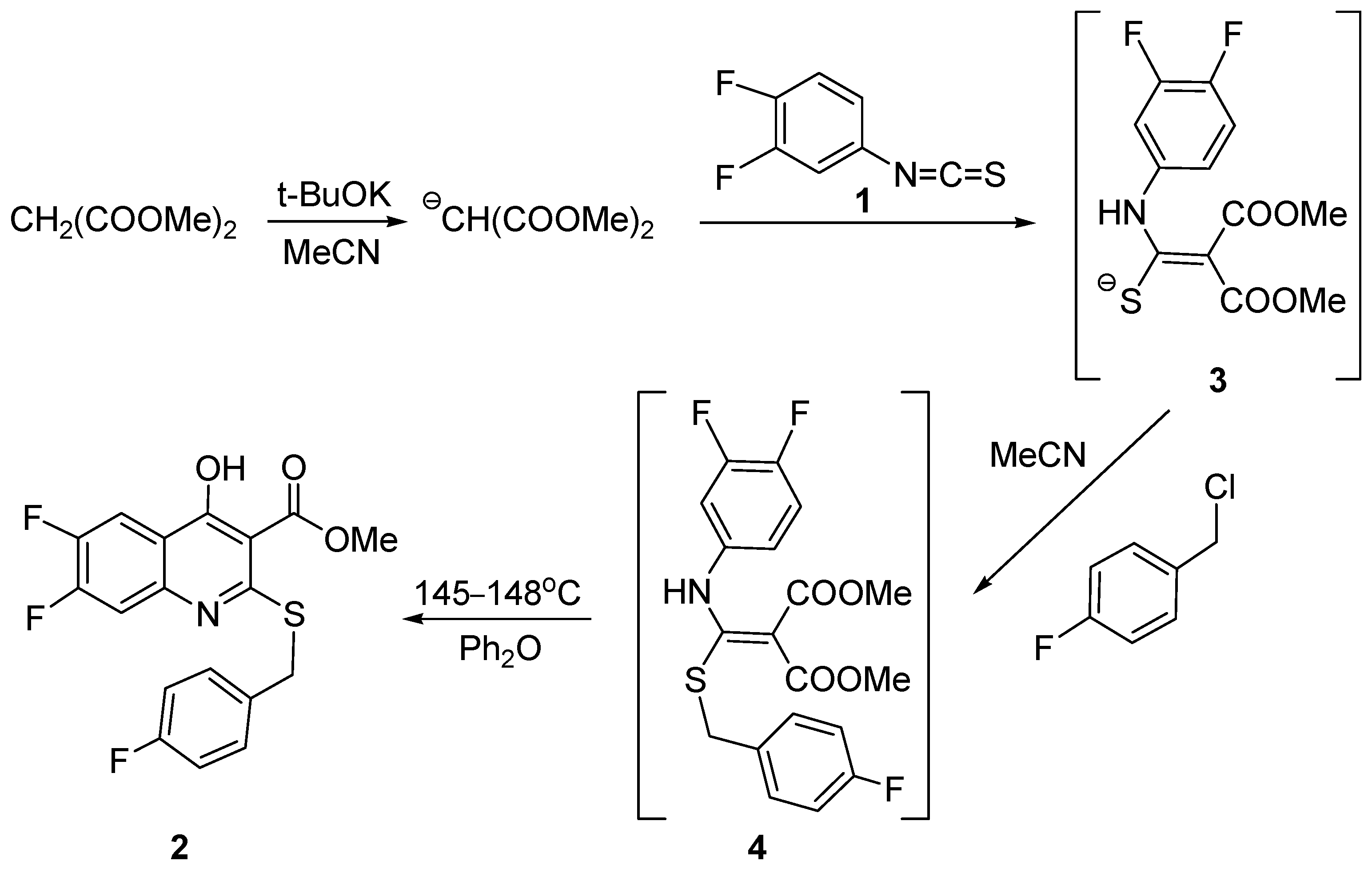
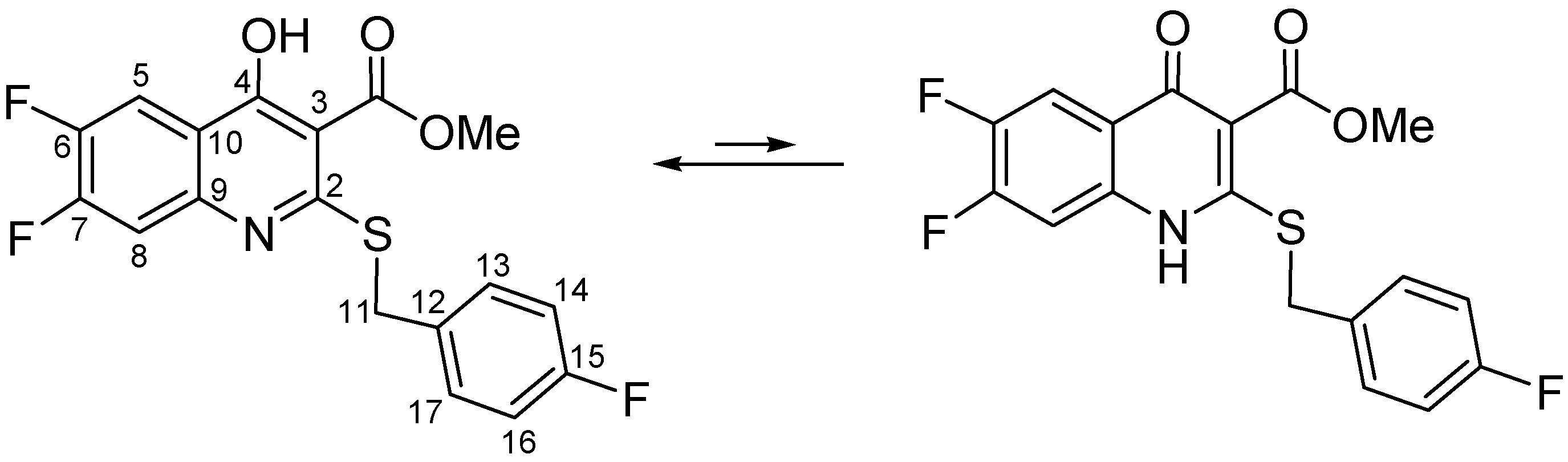
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of Compound 2
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Jackson, M.A. The use of systemic and topical fluoroquinolones. Pediatrics 2011, 128, e1034–e1045. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Daneshtalab, M. Nonclassical Biological Activities of Quinolone Derivatives. J. Pharm. Pharmaceut. Sci. 2012, 15, 52–72. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Fontaine, S.; Adam, H.; Schurek, K.; Mayer, M.; Noreddin, A.M.; Gin, A.S.; Rubinstein, E.; Hoban, D.J. A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections. Treat. Respir. Med. 2006, 5, 437–465. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Slejko, J.F.; Heil, E.L.; Seo, D.; Qato, D.M. Impact of the US Food and Drug Administration warning regarding increased risk of aortic aneurysms or aortic dissections on fluoroquinolone prescribing trends. BMJ Open Qual. 2024, 13, e002925. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Polyzos, K.A.; Sgouros, K.; Falagas, M.E. Prulifloxacin: A review focusing on its use beyond respiratory and urinary tract infections. Int. J. Antimicrob. Agents 2011, 37, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, R.; Rodloff, A.C. Activities of Quinolones Against Obligately Anaerobic Bacteria. Anti-Infect. Agents Med. Chem. 2007, 6, 49–56. [Google Scholar] [CrossRef]
- Shin, Y.S.; Lee, J.Y.; Jeon, S.; Myung, S.; Gong, H.J.; Kim, S.; Kim, H.R.; Jeong, L.S.; Park, C.M. Discovery of 2-aminoquinolone acid derivatives as potent inhibitors of SARS-CoV-2. Bioorg. Med. Chem. Lett. 2023, 85, 129214. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Verma, N.K.; Jain Anshul, K.; Srinivasan Chidambaram, V.; Wadhwa, L. An Improved Method for the Preparation of Highly Pure. Prulifloxacin. Patent WO2009093268, 30 June 2009. [Google Scholar]
- Liu, R.; Liu, J.; Su, Q.; Li, Q.; Li, R.; Chen, L.; Luo, C. New Method for Synthesizing Prulifloxacin from [(3,4-Difluorophenyl)amino](ethylthio)methylenemalonic Acid Diethyl Ester in Ionic. Liquid. Patent CN101565428, 28 October 2009. [Google Scholar]
- Lin, K.; Li, X.; Tao, Y.; Wang, Y. Method for Preparing. Prulifloxacin. Patent CN101857602, 13 October 2010. [Google Scholar]
- Zhao, J.; Cheng, H.; Sun, Y.; Chen, Y.; Wang, Q.; Yu, X.Y.; Xu, M.; Wang, L.; Li, X. Preparation of Oxazinoquinoline Compounds as Stimulator of Interferon Genes (STING) Modulators, and Compositions and Methods. Thereof. Patent WO2023158862, 21 February 2023. [Google Scholar]
- Rakitin, O.A. Strategies for the annulation of five-membered sulfur-nitrogen rings to benzene and heterocycles. Adv. Heterocycl. Chem. 2024, 142, 227–281. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Belovezhets, L.A.; Amosova, S.V. A Novel Family of [1,4]Thiazino[2,3,4-ij]quinolin-4-ium Derivatives: Regioselective Synthesis Based on Unsaturated Heteroatom and Heterocyclic Compounds and Antibacterial Activity. Molecules 2021, 26, 5579. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Efficient Regioselective Synthesis of Novel Condensed Sulfur–Nitrogen Heterocyclic Compounds Based on Annulation Reactions of 2-Quinolinesulfenyl Halides with Alkenes and Cycloalkenes. Molecules 2021, 26, 4844. [Google Scholar] [CrossRef] [PubMed]
- Unver, Y.; Celik, F.; Aydin, A.; Suleymanoglu, N.; Ustabas, R.; Guler, H.I.; Bektas, K.I. New 1,2,4-triazol-3-one derivatives with 4-fluorobenzene: Synthesis, characterization, DFT, antimicrobial-antiproliferative activities and molecular docking study. J. Mol. Struct. 2024, 1305, 137806. [Google Scholar] [CrossRef]
- Kumar, V.; Rai, V.M.; Udupi, V.; Shivalingegowda, N.; Pai, V.R.; Krishnappagowda, L.N.; Poojary, B. Synthesis, crystal structure, anticancer and molecular docking studies ofquinolinone-thiazolidinone hybrid molecules. J. Iran. Chem. Soc. 2022, 19, 793–808. [Google Scholar] [CrossRef]
- Ahadi, H.; Shokrzadeh, M.; Hosseini-Khah, Z.; Ghassemi barghi, N.; Ghasemian, M.; Emadi, E.; Zargari, M.; Razzaghi-Asl, N.; Emami, S. Synthesis and biological assessment of ciprofloxacin-derived 1,3,4-thiadiazoles as anticancer agents. Bioorg. Chem. 2020, 105, 104383. [Google Scholar] [CrossRef] [PubMed]
- Shinkre, B.A.; Raisch, K.P.; Fan, L.; Velu, S.E. Synthesis and antiproliferative activity of benzyl and phenethyl analogs of makaluvamines. Bioorg. Med. Chem. 2008, 16, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
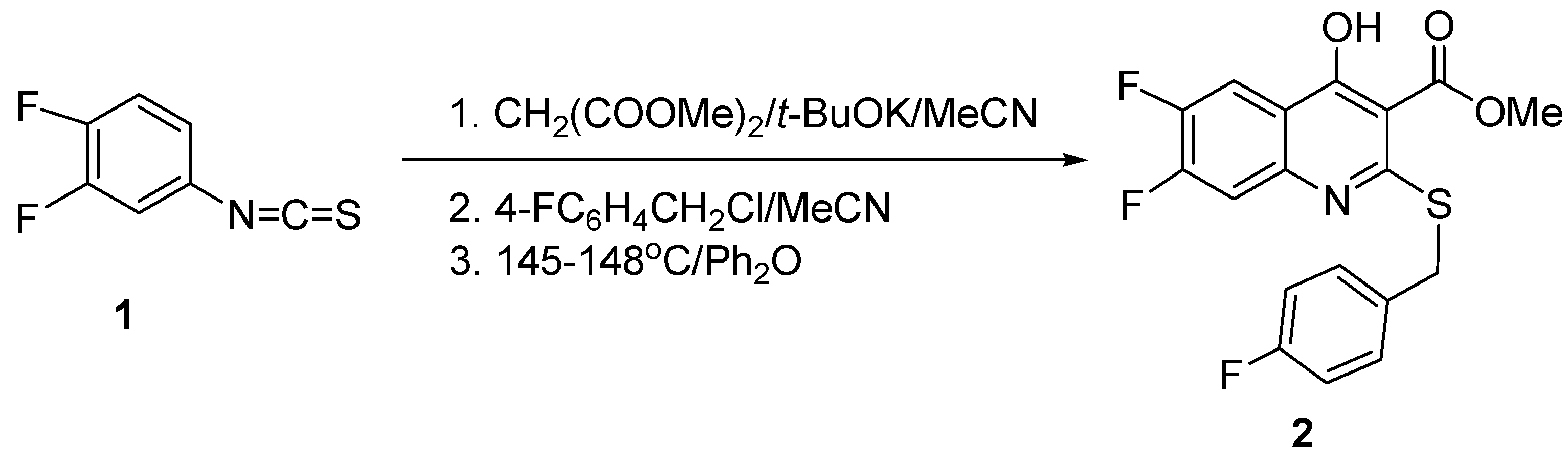
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.A.; Novokshonova, I.A.; Musalov, M.V.; Amosova, S.V.; Rakitin, O.A. Methyl 6,7-Difluoro-2-[(4-fluorobenzyl)sulfanyl]-4-hydroxyquinoline-3-carboxylate. Molbank 2024, 2024, M1889. https://doi.org/10.3390/M1889
Potapov VA, Novokshonova IA, Musalov MV, Amosova SV, Rakitin OA. Methyl 6,7-Difluoro-2-[(4-fluorobenzyl)sulfanyl]-4-hydroxyquinoline-3-carboxylate. Molbank. 2024; 2024(4):M1889. https://doi.org/10.3390/M1889
Chicago/Turabian StylePotapov, Vladimir A., Irina A. Novokshonova, Maxim V. Musalov, Svetlana V. Amosova, and Oleg A. Rakitin. 2024. "Methyl 6,7-Difluoro-2-[(4-fluorobenzyl)sulfanyl]-4-hydroxyquinoline-3-carboxylate" Molbank 2024, no. 4: M1889. https://doi.org/10.3390/M1889
APA StylePotapov, V. A., Novokshonova, I. A., Musalov, M. V., Amosova, S. V., & Rakitin, O. A. (2024). Methyl 6,7-Difluoro-2-[(4-fluorobenzyl)sulfanyl]-4-hydroxyquinoline-3-carboxylate. Molbank, 2024(4), M1889. https://doi.org/10.3390/M1889







