Synthesis of Novel Sulfonamide Derivatives Featuring 1-(Methylsulfonyl)-4-(2,3,4-Trimethoxybenzyl)Piperazine Core Structures
Abstract
1. Introduction
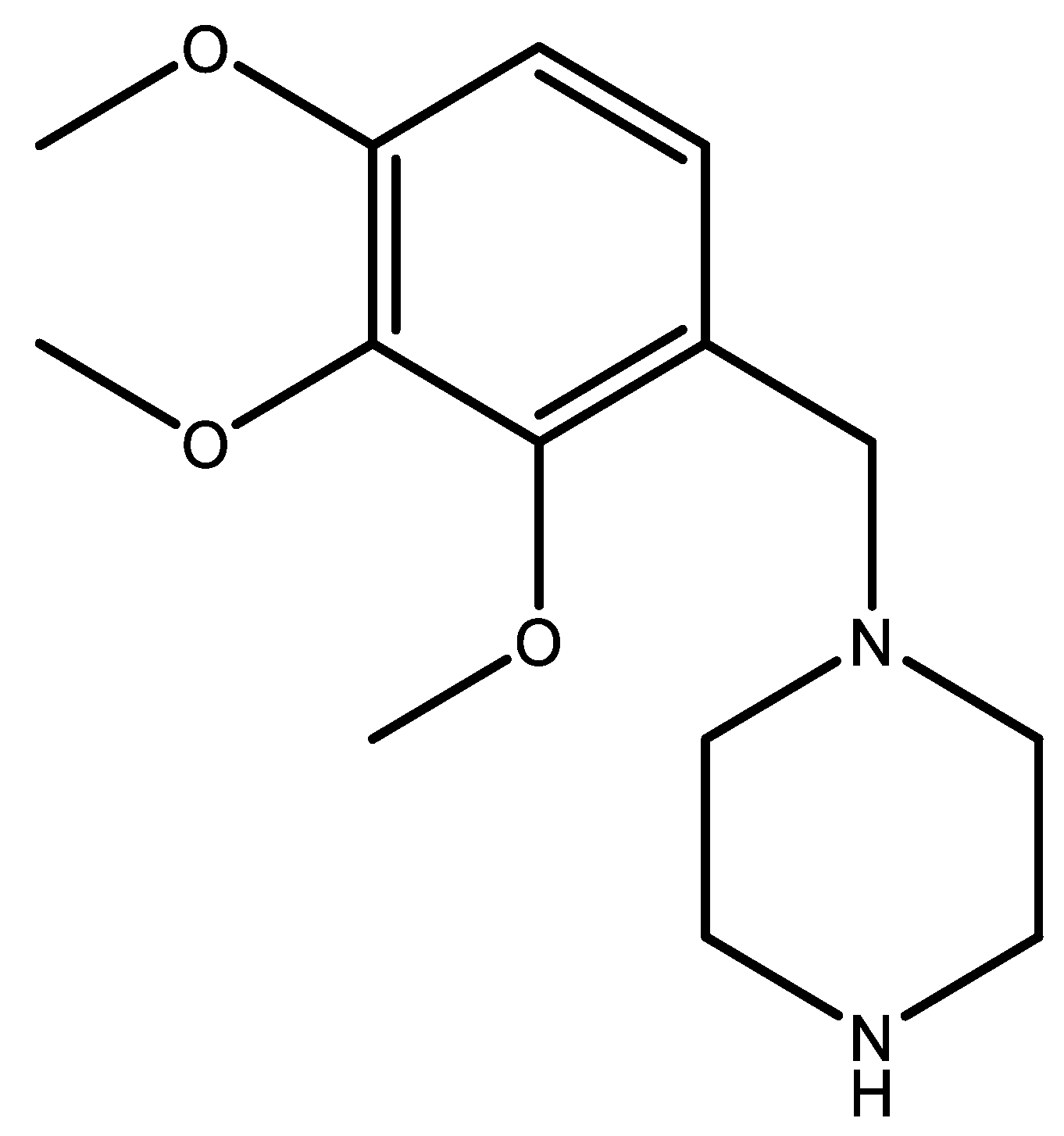
2. Results and Discussion
3. Materials and Methods
Synthetic Procedure
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onay-Besikci, A.; Ozkan, S. Trimetazidine revisited: A comprehensive review of the pharmacological effects and analytical techniques for the determination of trimetazidine. Cardiovasc. Ther. 2008, 26, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.; Marzilli, M. Metabolic therapy in the treatment of ischaemic heart disease: The pharmacology of trimetazidine. Fundam. Clin. Pharmacol. 2003, 17, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.; Al-Gareeb, A. Central Beneficial Effects of Trimetazidine on Psychomotor Performance in Normal Healthy Volunteers. Adv. Biomed. Res. 2017, 6, 6–69. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency Recommends Restricting Use of Trimetazidine-Containing Medicines Reference Number: EMA/CHMP/417861/2012 European Medicines Agency Recommends Restricting Use of Trimetazidine-Containing Medicines (europa.eu). Available online: https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-restricting-use-trimetazidine-containing-medicines (accessed on 24 July 2024).
- Zhang, W.; You, B.; Qi, D.; Qui, L.; Ripley-Gonzalez, J.; Zheng, F.; Fu, S.; Li, C.; Dun, Y.; Liu, S. Trimetazidine and Exercise provide comparable improvements to high fat diet-induced muscle dysfunction through enhancement of mitochondrial quality control. Sci. Rep. 2021, 11, 19116. [Google Scholar] [CrossRef] [PubMed]
- Ovung, A.; Bhattacharyya, A. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Pușcaș, A.; Ștefănescu, R.; Vari, C.-E.; Ősz, B.-E.; Filip, C.; Bitzan, J.K.; Buț, M.-G.; Tero-Vescan, A. Biochemical Aspects That Lead to Abusive Use of Trimetazidine in Performance Athletes: A Mini-Review. Int. J. Mol. Sci. 2024, 25, 1605. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, G.; Koch, A.; Orlovius, A.-K.; Guddat, S.; Thomas, A.; Schänzer, W.; Thevis, M. Doping control analysis of trimetazidine and characterization of major metabolites using mass spectrometric approaches. Drug Test. Anal. 2014, 6, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Igwe, C.N.; Okoro, U.C. Synthesis, Characterization, and Evaluation for Antibacterial and Antifungal Activities of N-Heteroaryl Substituted Benzene Sulphonamides. Org. Chem. Int. 2014, 2014, 419518. [Google Scholar] [CrossRef][Green Version]
- Yousef, F.; Mansour, O.; Herbali, J. Sulfonamides: Historical discovery development (Structure-activity relationship notes). In-vitro In-Vivo In-Silico J. 2018, 1, 1–15. [Google Scholar]
- Zawodniak, A.; Lochmatter, P.; Beeler, A.; Pichler, W. Cross-Reactivity in Drug Hypersensitivity Reactions to Sulfasalazine and Sulfamethoxazole. Int. Arch. Allergy Immunol. 2010, 153, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miyazaki, M.; Mashima, K.; Takagi, S.; Hara, S.; Kamimura, H.; Jimi, S. The Effects of Silver Sulfadiazine on Methicillin-Resistant Staphylococcus aureus Biofilms. Microorganisms 2020, 8, 1551. [Google Scholar] [CrossRef] [PubMed]
- Seneca, H. Long-Acting Sulfonamides in Urinary Tract Infections. JAMA 1966, 198, 975–980. [Google Scholar] [CrossRef]
- Wiedemann, B.; Heisig, A.; Heisig, P. Uncomplicated Urinary Tract Infections and Antibiotic Resistance—Epidemiological and Mechanistic Aspects. Antibiotics 2014, 3, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Chio, L.-C.; Bolyard, L.; Nasr, M.; Queener, S. Identification of a Class of Sulfonamides Highly Active against Dihydropteroate Synthase from Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob. Agents Chemother. 1996, 40, 727–733. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Zach, S.; Wang, X.; Potluri, L.-P.; Neville, A.; Vennerstrom, J.; Davis, P. Review of Experimental Compounds Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2016, 60, 7017–7034. [Google Scholar] [CrossRef] [PubMed]
- Alkhzem, A.; Woodman, T.; Blagbrough, I. Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef] [PubMed]
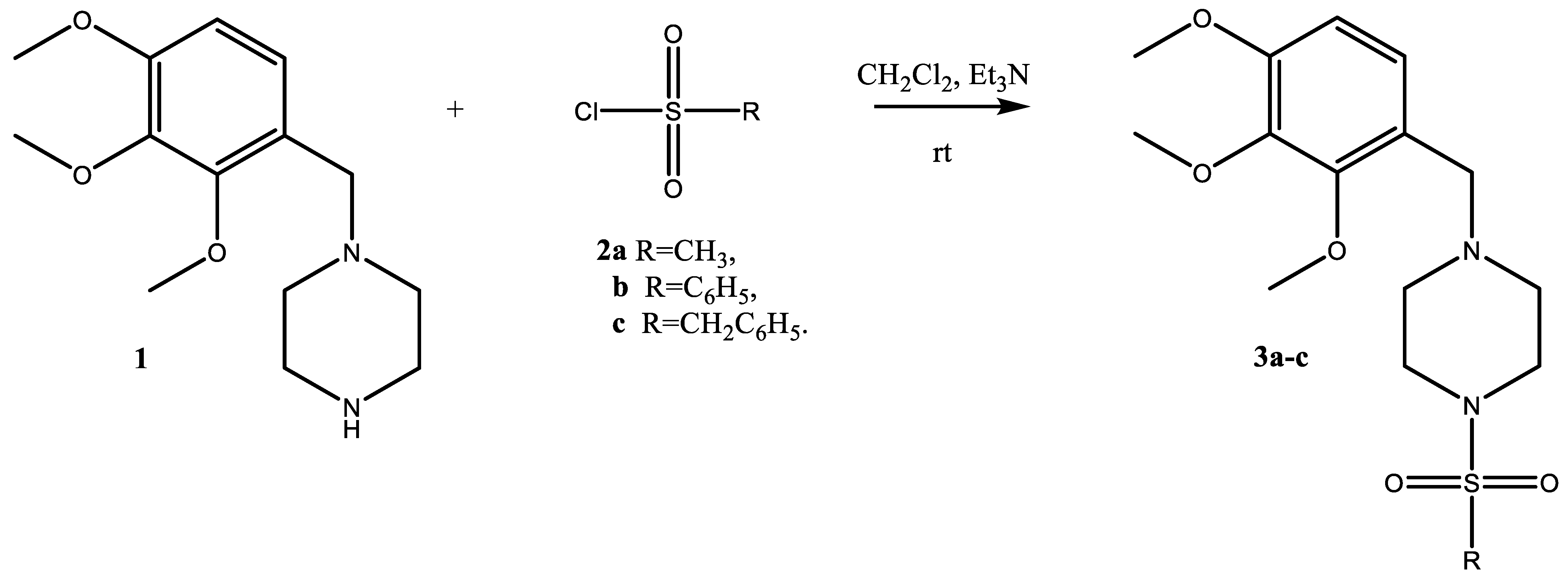
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, I.; Manolov, S.; Bojilov, D.; Dimitrova, D.; Nedialkov, P. Synthesis of Novel Sulfonamide Derivatives Featuring 1-(Methylsulfonyl)-4-(2,3,4-Trimethoxybenzyl)Piperazine Core Structures. Molbank 2024, 2024, M1879. https://doi.org/10.3390/M1879
Ivanov I, Manolov S, Bojilov D, Dimitrova D, Nedialkov P. Synthesis of Novel Sulfonamide Derivatives Featuring 1-(Methylsulfonyl)-4-(2,3,4-Trimethoxybenzyl)Piperazine Core Structures. Molbank. 2024; 2024(3):M1879. https://doi.org/10.3390/M1879
Chicago/Turabian StyleIvanov, Iliyan, Stanimir Manolov, Dimitar Bojilov, Diyana Dimitrova, and Paraskev Nedialkov. 2024. "Synthesis of Novel Sulfonamide Derivatives Featuring 1-(Methylsulfonyl)-4-(2,3,4-Trimethoxybenzyl)Piperazine Core Structures" Molbank 2024, no. 3: M1879. https://doi.org/10.3390/M1879
APA StyleIvanov, I., Manolov, S., Bojilov, D., Dimitrova, D., & Nedialkov, P. (2024). Synthesis of Novel Sulfonamide Derivatives Featuring 1-(Methylsulfonyl)-4-(2,3,4-Trimethoxybenzyl)Piperazine Core Structures. Molbank, 2024(3), M1879. https://doi.org/10.3390/M1879








