Abstract
Bromination of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical with bromine or N-bromo-succinimide (NBS) affords a complex mixture of bromo- and nitro-derivatives of the starting material. In this study, by chromatographic separation, most of the reaction products were isolated. Suitable crystals for X-ray measurements were obtained and characterized for the compounds 2-p-bromophenyl-2-phenyl-1-picrylhydrazyl free radical (Br-DPPH), 2-p-bromophenyl-2-phenyl-1-picrylhydrazine (Br-DPPH-H), and 2,2-(p-bromophenyl)-1-(2-bromo-4,6-dinitrophenyl)hydrazine (Br2-DPPBr-H).
1. Introduction
2,2-diphenyl-1-picrylhydrazyl (DPPH) is a commercially available stable free radical that is used as the main reagent in total antioxidant assays (TAC; DPPH method [1,2]) and as the electron paramagnetic resonance standard [3]. Besides these main uses, DPPH showed unique acid–base, redox, and chromogenic properties [4], which have all been reviewed recently [5].
Bromination of DPPH or its reduced counterpart 2,2-diphenyl-1-picrylhydrazine (DPPH-H) leads to a mixture of compounds from which some bromo- or nitro-derivatives can be isolated [6,7]. Although the bromo-derivatives are normally expected to be obtained, nitro-derivatives appear as by-products in the bromination process, which follows a radical mechanism (Figure 1). As a consequence, ipso-substitution of the nitro-group with a bromine atom is possible [8].

Figure 1.
Reaction of DPPH (or DPPH-H) with bromine (or NBS).
While the crystal structures of the parent DPPH and other derivatives have been reported in the literature [9,10,11,12,13,14], the crystal structures of the compounds 2-p-bromophenyl-2-phenyl-1-picrylhydrazyl free radical (Br-DPPH), 2-p-bromophenyl-2-phenyl-1-picrylhydrazine (Br-DPPH-H), and 2,2-(p-bromophenyl)-1-(2-bromo-4,6-dinitrophenyl)hydrazine (Br2-DPPBr-H) have not been described until now. Therefore, this work uncovers novel structural insights into such compounds.
2. Results and Discussion
Synthesis of the above-mentioned brominated compounds can be achieved in the following ways, as literature data have shown [5,6,7,8]: (i) compounds Br-DPPH-H and Br-DPPH can be obtained through direct syntheses, starting from the corresponding bromo-diphenylamines, following the well-known procedures involved in the synthesis of DPPH (nitrozation, reduction, coupling with picryl chloride and oxidation); (ii) the compounds Br-DPPH-H, Br-DPPH, and Br2-DPPBr-H can be obtained by bromination with different reagents (bromine, hydrobromic acid, or NBS) of DPPH or DPPH-H. In the first case (step-by-step synthesis), all reactions occur with high yields, while in the second case, a complex mixture of derivatives is obtained (Figure 1), requiring separation.
In order to obtain all the required bromo-derivatives via direct synthesis, we followed the procedure described by Weil [6,8], although the separation of such compounds is difficult and can be achieved only by chromatographic means. It is worth mentioning that the nitro-derivatives denoted as NO2-DPPH and (NO2)2-DPPH-H (Figure 1) are always found in this complex mixture. As mentioned before, they are formed in a radical + radical reaction between DPPH and nitrogen dioxide (this is formed via an ipso-substitution reaction, undoubtedly proven using 15N labeling [15]). Instead of a simple para-phenyl bromination, the complex mixture of reaction products indicates a more multifaceted reaction pathway, in which the nitration products observed in the literature are formed in high yields [6]. Additionally, the reaction conditions, such as temperature and the rate of bromine addition, significantly influence the product yields, highlighting the sensitivity of the bromination process to experimental parameters [8]. After careful separation of the reaction products, slow evaporation of the solvent afforded suitable crystals for X-ray analysis for the bromo-derivatives Br-DPPH-H, Br-DPPH, and Br2-DPPBr-H. Unfortunately, for Br2-DPPH-H, no good crystals were formed and therefore, for this compound, no structural details were obtained.
The compound denoted as Br-DPPH-H (Figure 1) crystallizes in the orthorhombic P212121 space group with one molecule in the asymmetric unit. The molecular structure of this hydrazine is presented in Figure 2. The N1-N2 bond length within the hydrazine moiety is 1.413(8) Å, while the N-C bond lengths with the aromatic substituents are as follows: N1-C1 = 1.448(10), N1-C7 = 1.443(10) and N2-C13 = 1.349(9) Å. Within the picryl fragment, two of the nitro groups are almost coplanar with the benzene ring (O1-N3-O2 and O3-N4-O4). The O5-N5-O6 nitro group has a dihedral angle of 58.7° with the mean plan of the benzene ring. The hydrazine hydrogen atom, H2N, is involved in an intramolecular hydrogen interaction with the O1 oxygen atom of the O1-N3-O2 nitro group. The (N2)-H2N···O1 distance is 2.027(7) Å and the N2-H2N···O1 angle is 123.7(5)°.
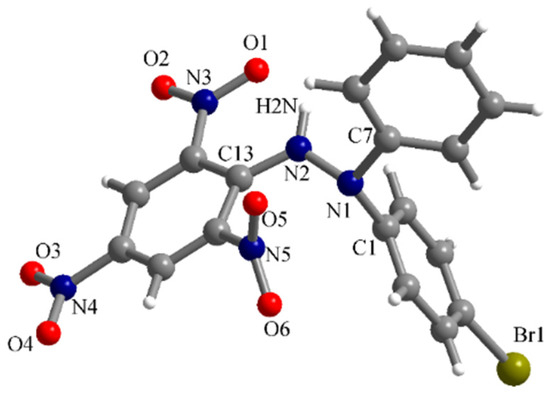
Figure 2.
Perspective view of the molecular structure of Br-DPPH-H determined by X-ray diffraction.
The analysis of the packing diagrams of Br-DPPH-H shows a “herringbone” organization of the molecules in the crystallographic bc plane (Figure 3). Along the crystallographic a axis, the molecules generate supramolecular columns by halogen–π interactions established between the bromine atoms and benzene rings of the picryl moieties of neighboring molecules (Figure 3b). The Br1···C17’ distance is 3.382(8) Å (symmetry code: ’ = 0.5 + x, 0.5-y, 1-z).
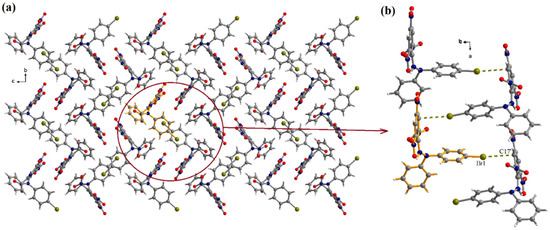
Figure 3.
View of the packing diagram in crystal Br-DPPH-H along the crystallographic a axis (a) and details of the halogen–π interactions (b). Symmetry code: ’ = 0.5 + x, 0.5-y, 1-z.
The stable free radical Br-DPPH also crystallizes in an orthorhombic space group (Pca21) but the asymmetric unit contains two independent molecules (Figure 4). In this radical, the N-N bond lengths are N1-N2 = 1.343(9) and N6-N7 = 1.344(9) Å. The N-C bond lengths with the aromatic substituents are N1-C1 = 1.414(11), N1-C7 = 1.455(11), N2-C13 = 1.367(10), N6-C19 = 1.423(11), N6-C25 = 1.455(11) and N7-C31 = 1.348(11) Å. Within the picryl fragments, only the nitro groups from para positions are coplanar with the benzene rings, revealing the influence of the intramolecular hydrogen interaction in the hydrazine precursor, as the nitro group from the ortho position involved in hydrogen bonding is also coplanar with the benzene ring.
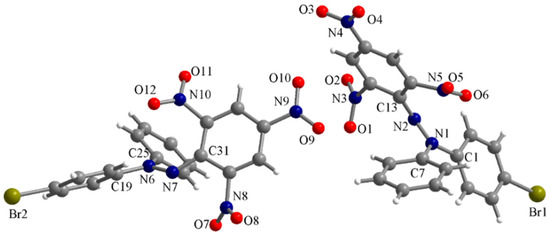
Figure 4.
Perspective view of the asymmetric unit in the Br-DPPH crystal.
A view of the packing diagram in the crystal structure of the free radical Br-DPPH along the crystallographic b axis is presented in Figure 5a. The halogen–π interactions in crystal 2 are longer, where Br1···C20’ = 3.718(9) Å (symmetry code: ’ = 0.5-x, y, −0.5 + z).

Figure 5.
View of the packing diagram in Br-DPPH crystal along the crystallographic b axis (a) and details of the halogen–π interactions (b). Symmetry code: ’ = 0.5-x, y, −0.5 + z.
Finally, the hydrazine derivative Br2-DPPBr-H crystallizes in the monoclinic space group P21/c along with dichloromethane solvent molecules in a 1:1 molar ratio (Figure 6). The N1-N2 bond length within the hydrazine moiety is 1.395(7) Å and the N-C bond lengths with the aromatic substituents are as follows: N1-C7 = 1.442(7), N1-C13 = 1.429(8) and N2-C1 = 1.368(8) Å. In the bromo-dinitrobenzene fragment, the planes of nitro groups have a mean plan of benzene ring dihedral angles of 11.2° (O3-N4-O4) and 60.4° (O1-N3-O2). The hydrogen atom of the hydrazine is positioned near the Br1 atom.

Figure 6.
Perspective view of the asymmetric unit in crystal Br2-DPPBr-H.
The bromines Br2 and Br3 establish short interactions with nitro groups belonging to neighboring molecules of the compound Br2-DPPBr-H, Br2···O1’ = 3.219(6) and Br3···O2” = 3.204(6) Å (Figure 7, symmetry codes: ’ = 1 + x, 0.5-y, 0.5 + z; ” = x, y, −1 + z). These interactions generate supramolecular 2D arrangements of the molecules.
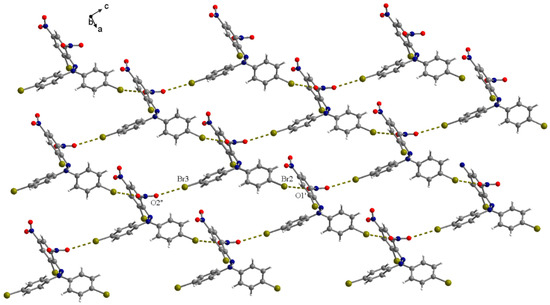
Figure 7.
View of the packing diagram in Br2-DPPBr-H crystal along the crystallographic b axis showing the bromo-nitro contacts.
A compilation of general data for the crystals obtained is presented in Table 1.

Table 1.
Crystallographic data, details of data collection and structure refinement parameters.
3. Materials and Methods
3.1. Synthesis
The general procedure [6,7,8] for the synthesis of the compounds shown in Figure 1 is summarized as follows. First, 1 g of DPPH dissolved in 100 mL dichloromethane (DCM) was treated under stirring at room temperature with a 10% bromine solution in the same solvent. The addition was performed slowly (about 2 h) and then the mixture was left until the next day, when the solution was vigorously shaken with 100 mL of water containing some sodium thiosulfate (3–5 g) in order to remove the bromine in excess. The organic solvent was collected and dried over anhydrous sodium sulphate. After filtration and evaporation of the solvent, the residue could then be chromatographed on an alumina or silica gel column using a mixture of hexanes and DCM as the eluent. From our experience, basic alumina is more effective, and the order of elution is usually unreacted DPPH-H, followed by brominated derivates and the nitrated compound NO2-DPPH-H, as literature data have shown [6]. However, if silica gel is used as the stationary phase, the order of elution is different, where the brominated derivatives leave the chromatographic column first, followed by the unreacted starting material and nitrated compounds. It is necessary to test the purity of the separated compounds by TLC for all the derivatives, as the retention values are very close; better separation can be performed on preparative TLC (or using the free radicals obtained after oxidation). If the purity of the separated compounds is satisfactory, slow evaporation of the solvent affords suitable crystals for the bromo-derivatives Br-DPPH-H, Br-DPPH, and Br2-DPPBr-H. The yields were found to be similar with literature data [6], meaning 5–6% unreacted DPPH-H, 63–67% Br-DPPH-H, 10% Br2-DPPH-H, and 5% Br2-DPPBr-H.
To avoid working with bromine (which is difficult to handle), a similar synthesis procedure can be performed using NBS and DPPH (or DPPH-H), as shown in Figure 1. Thus, instead of the bromine solution, 0.5 g of solid NBS was used, following the exact procedure and work-up as described above. In both cases, the same brominated compounds were obtained and their purities were assessed by NMR, which were perfectly in line with the literature data [6,7,8].
3.2. X-ray Crystallographic Analysis
X-ray diffraction data were collected at 293 K using a Rigaku XtaLAB Synergy-S diffractometer operating with a Mo-Kα (λ = 0.71073 Å) micro-focus sealed X-ray tube (Rigaku Corporation, Tokyo, Japan). The structures were solved by direct methods and refined by full-matrix least squares techniques based on F2. The non-H atoms were refined with anisotropic displacement parameters and hydrogen atoms were introduced at calculated positions (riding model), except for the hydrazine hydrogen atom of the compound Br2-DPPBr-H·DCM. Calculations were performed using the SHELX-2018 crystallographic software package. A summary of the crystallographic data and the structure refinement for the crystals are given in Table 1. The CCDC reference numbers are as follows: 2328957-2328959.
4. Conclusions
The crystal structures of three bromo-derivatives of the well-known stable free radical DPPH were revealed by X-ray diffraction. The data obtained complete the structural characterization of hydrazyls and hydrazines, expanding the available databases.
Supplementary Materials
The mol file, cif file and check cif report are the Supplementary Materials.
Author Contributions
Conceptualization, P.I.; methodology, all authors; software, A.M.M. and P.I.; validation and formal analysis, all authors; writing—original draft preparation, A.M.M. and P.I.; writing—review and editing, all authors; supervision, P.I.; project administration, P.I.; funding acquisition, A.F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CSUD-UB.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2010, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Zhuang, J.; Guzzetta, F.; Lynch, J.; Angerhofer, A.; Cao, Y.C. Synthesis of water-soluble 2,2-diphenyl-1-picrylhydrazyl nanoparticles: A new standard for electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 2009, 131, 12542–12543. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, B.; Lete, C.; Popescu, C.; Matache, M.; Paun, A.; Madalan, A.; Ionita, P. Synthesis and spectral comparison of electronic and molecular properties of some hydrazines and hydrazyl free radicals. Arkivoc 2020, vi, 1–10. [Google Scholar] [CrossRef]
- Ionita, P. The Chemistry of DPPH· Free Radical and Congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.F.; Quail, J.W.; Weil, J.A. The reaction between 2,2-diphenyl-1-picrylhydrazyl and bromine. Can. J. Chem. 1980, 58, 723–726. [Google Scholar] [CrossRef]
- Ionita, P. The reaction between DPPH free stable radical and N-bromosuccinimide. South Braz. J. Chem. 1998, 6, 101–106. [Google Scholar] [CrossRef]
- Currie, P.F.; Quail, J.W.; Rusk, A.C.M.; Weil, J.A. A study of Br/NO2 substitution reactions and nuclear magnetic resonance of 2,2-diaryl-1-picrylhydrazines. Can. J. Chem. 1983, 61, 1760–1765. [Google Scholar] [CrossRef]
- Williams, D.E. Crystal structure of 2,2-diphenyl-1-picrylhydrazyl free radical. J. Am. Chem. Soc. 1967, 89, 4280–4287. [Google Scholar] [CrossRef]
- Zilic, D.; Pajic, D.; Juric, M.; Molcanov, K.; Rakvin, B.; Planinic, P.; Zadro, K. Single crystals of DPPH grown from diethyl ether and carbon disulfide solutions- Crystal structures, IR, EPR and magnetization studies. J. Magn. Reson. 2010, 207, 34–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Barton, R.J.; Robertson, B.E. Crystal and molecular structure of 9-(2,4,6-trinitroanilin)-carbazole. Can. J. Chem. 1987, 65, 1322–1326. [Google Scholar] [CrossRef]
- Gopal, R.; Robertson, B.E.; Weil, J.A. The crystal structure of potassium 1-(N,N-diphenylhydrazono)-2,4,6-trinitrobenzenide. Can. J. Chem. 1983, 61, 2735–2739. [Google Scholar] [CrossRef]
- Wang, H.; Barton, R.J.; Robertson, B.E. Structural studies of 2,2-diphenyl-1-picrylhydrazine: A clathrate forming compound. J. Incl. Phenom. Macrocycl. Chem. 1991, 10, 203–217. [Google Scholar] [CrossRef]
- Kiers, C.T.; de Boer, J.L.; Olthof, R.; Spek, A.L. The crystal structure of a 2,2-diphenyl-1-picrylhydrazyl (DPPH) modification. Acta Crystal. B Struct. Crystal Chem. 1976, 32 B, 2297–2305. [Google Scholar] [CrossRef]
- Ionita, P.; Caproiu, M.T.; Luca, C.; Constantinescu, T.; Caldararu, H.; Balaban, A.T. Selective (15N) nitration of 2,2-diphenyl-1-(2,4- or 2,6-dinitrophenyl)-hydrazines or -hydrazyls. J. Label. Comp. Radiopharm. 1998, 41, 791–799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).