1-(Dicyanomethylene)-3-hydroxy-1H-indene-2-carboxylic Acid
Abstract
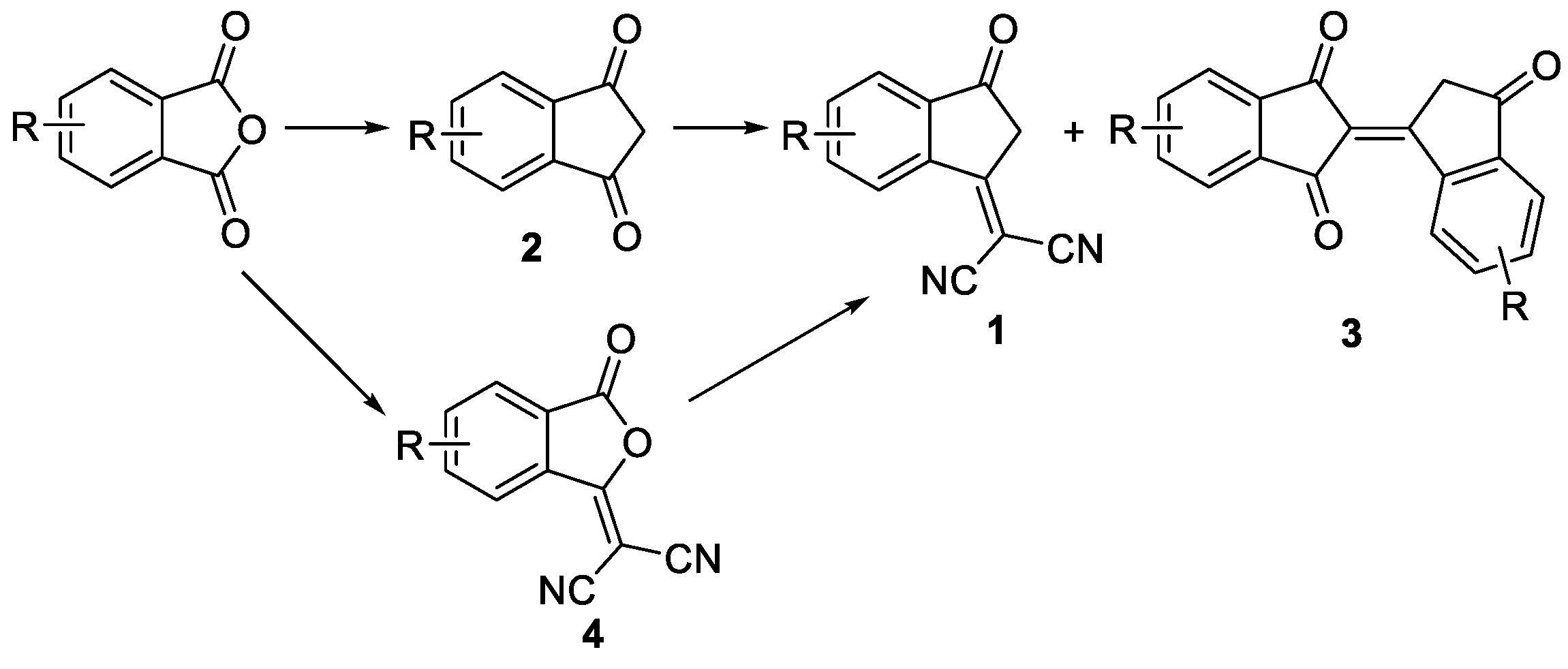
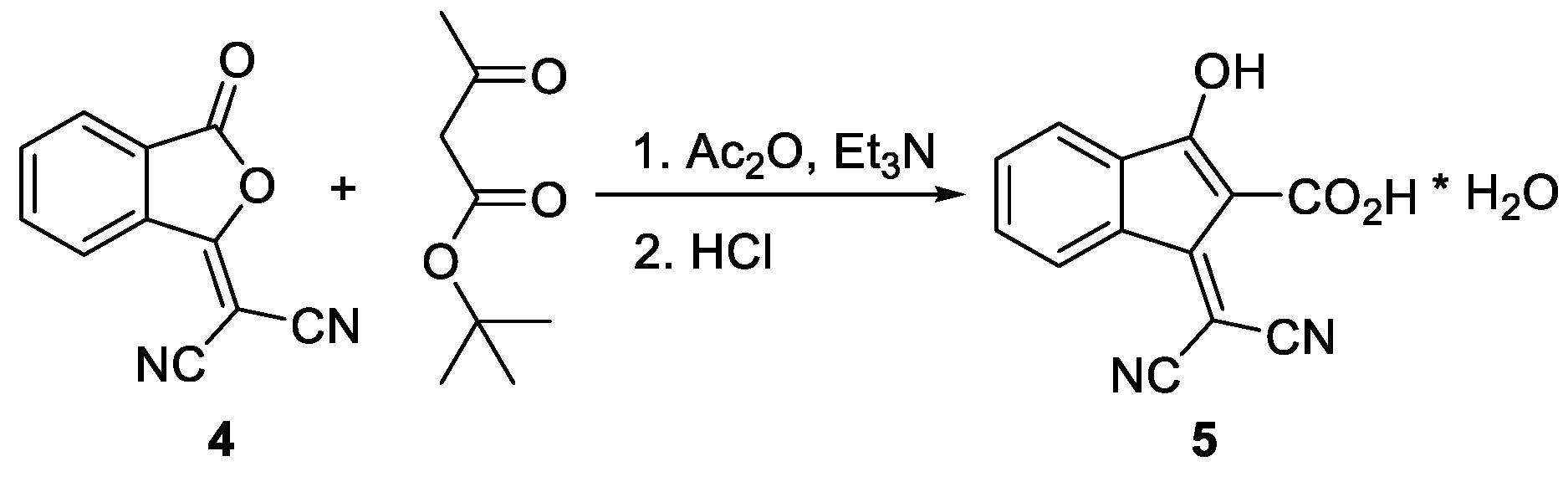
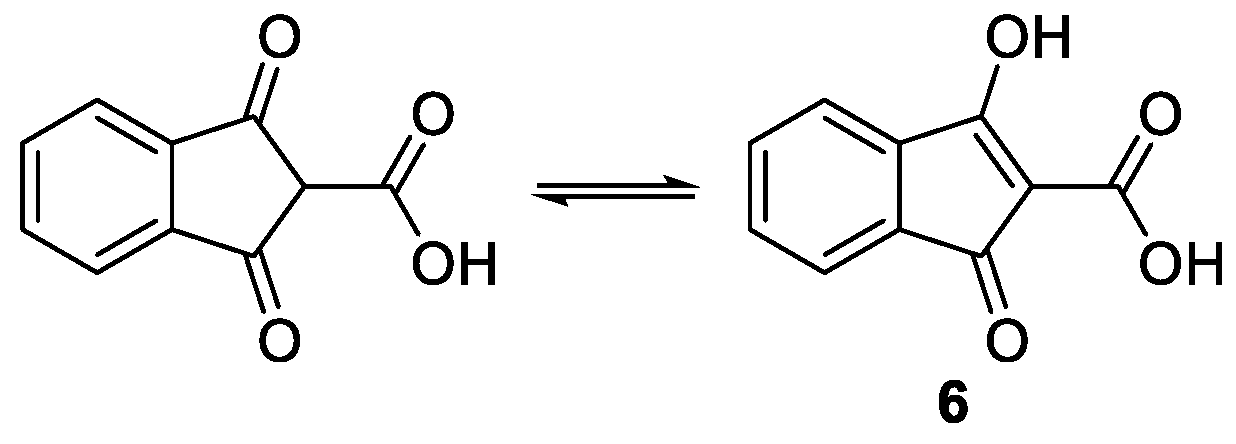
1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deibel, C.; Dyakonov, V. Polymer–Fullerene Bulk Heterojunction Solar Cells. Rep. Prog. Phys. 2010, 73, 096401. [Google Scholar] [CrossRef]
- Jain, A.; Kothari, R.; Tyagi, V.V.; Kumar Rajamony, R.; Shakeel Ahmad, M.; Mohan Singh, H.; Raina, S.; Pandey, A.K. Advances in Organic Solar Cells: Materials, Progress, Challenges and Amelioration for Sustainable Future. Sustain. Energy Technol. Assess. 2024, 63, 103632. [Google Scholar] [CrossRef]
- Duan, T.; Feng, W.; Li, Y.; Li, Z.; Zhang, Z.; Liang, H.; Chen, H.; Zhong, C.; Jeong, S.; Yang, C.; et al. Electronic Configuration Tuning of Centrally Extended Non-Fullerene Acceptors Enabling Organic Solar Cells with Efficiency Approaching 19%. Angew. Chem. Int. Ed. 2023, 62, e202308832. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Bi, X.; Chen, H.; He, T.; Lin, Y.; Zhang, Y.; Ma, K.; Feng, W.; Ma, Z.; Long, G.; et al. A Rare Case of Brominated Small Molecule Acceptors for High-Efficiency Organic Solar Cells. Nat. Commun. 2023, 14, 4707. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, X.; Bao, H.; Feng, J.; Gao, X.; Liu, Z.; Ge, Z. Latest Progress on Fully Non-fused Electron Acceptors for High-performance Organic Solar Cells. Chin. Chem. Lett. 2023, 34, 107968. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Sun, G.; Li, Y. Organic Small Molecule Acceptor Materials for Organic Solar Cells. eScience 2023, 3, 100171. [Google Scholar] [CrossRef]
- Liu, H.; Dai, T.; Zhou, J.; Wang, H.; Guo, Q.; Guo, Q.; Zhou, E. The Development of A-DA’D-A Type Nonfullerene Acceptors Containing Non-Halogenated End Groups. Nano Res. 2023, 16, 12949–12961. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.; Chen, X.; Song, G.; Wan, X.; Kan, B.; Duan, T.; Knyazeva, E.A.; Rakitin, O.A.; Chen, Y. Side-Chain Modification of Non-Fullerene Acceptors for Organic Solar Cells with Efficiency over 18%. J. Mater. Chem. C 2023, 11, 6920–6927. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, T.; Zhang, C.; Yang, C. Side-Chain Engineering of Nonfullerene Small-Molecule Acceptors for Organic Solar Cells. Energy Environ. Sci. 2023, 16, 2732–2758. [Google Scholar] [CrossRef]
- Bello, K.A.; Cheng, L.; Griffiths, J. Near-Infrared Absorbing Methine Dyes Based on Dicyanovinyl Derivatives of Indane-1,3-Dione. J. Chem. Soc. Perkin Trans. 2 1987, 6, 815. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.; An, Q.; Jiang, M.; Mahmood, A.; Jee, M.H.; Bai, H.; Zhi, H.; Zhang, S.; Woo, H.Y.; et al. Regioisomer-Free Difluoro-Monochloro Terminal-based Hexa-Halogenated Acceptor with Optimized Crystal Packing for Efficient Binary Organic Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202209454. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra-Narrow Band Gap. Angew. Chem. Int. Ed. 2017, 56, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Planells, M.; Robertson, N. Naphthyl Derivatives Functionalised with Electron Acceptor Units—Synthesis, Electronic Characterisation and DFT Calculations. Eur. J. Org. Chem. 2012, 2012, 4947–4953. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.-K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; et al. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; An, R.; Cao, M.; Shu, H.; Wu, X.; Tong, H.; Wang, L. Nonfullerene Acceptors with Cyano-Modified Terminal Groups for Organic Solar Cells: Effect of Substitution Position on Photovoltaic Properties. Dye. Pigment. 2022, 206, 110661. [Google Scholar] [CrossRef]
- Bürckstümmer, H.; Tulyakova, E.V.; Deppisch, M.; Lenze, M.R.; Kronenberg, N.M.; Gsänger, M.; Stolte, M.; Meerholz, K.; Würthner, F. Efficient Solution-Processed Bulk Heterojunction Solar Cells by Antiparallel Supramolecular Arrangement of Dipolar Donor–Acceptor Dyes. Angew. Chem. Int. Ed. 2011, 50, 11628–11632. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.-T.; Péralta, S.; Dumur, F. Synthesis and Optical Properties of a Series of Push-Pull Dyes Based on Pyrene as the Electron Donor. Molecules 2023, 28, 1489. [Google Scholar] [CrossRef] [PubMed]
- Enchev, V.; Angelova, S.; Rogojerov, M.; Monev, V.; Wawer, I.; Tkaczyk, M.; Kostova, K. Solid-State Tautomerism in 2-Carboxyindan-1,3-Dione. J. Phys. Chem. A 2011, 115, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Angelova, S.; Enchev, V.; Kostova, K.; Rogojerov, M.; Ivanova, G. Theoretical and Spectroscopic Study of 2-Substituted Indan-1,3-Diones: A Coherent Picture of the Tautomeric Equilibrium. J. Phys. Chem. A 2007, 111, 9901–9913. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-Y.; Ren, L.-P.; Zou, M.-M.; Xu, Z.-P.; Shao, X.-S.; Xu, X.-Y.; Li, Z. Design, Synthesis and Insecticidal Activity of Spiro Heterocycle Containing Neonicotinoid Analogs. Chin. Chem. Lett. 2014, 25, 197–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usova, S.D.; Knyazeva, E.A.; Rakitin, O.A. 1-(Dicyanomethylene)-3-hydroxy-1H-indene-2-carboxylic Acid. Molbank 2024, 2024, M1871. https://doi.org/10.3390/M1871
Usova SD, Knyazeva EA, Rakitin OA. 1-(Dicyanomethylene)-3-hydroxy-1H-indene-2-carboxylic Acid. Molbank. 2024; 2024(3):M1871. https://doi.org/10.3390/M1871
Chicago/Turabian StyleUsova, Sofia D., Ekaterina A. Knyazeva, and Oleg A. Rakitin. 2024. "1-(Dicyanomethylene)-3-hydroxy-1H-indene-2-carboxylic Acid" Molbank 2024, no. 3: M1871. https://doi.org/10.3390/M1871
APA StyleUsova, S. D., Knyazeva, E. A., & Rakitin, O. A. (2024). 1-(Dicyanomethylene)-3-hydroxy-1H-indene-2-carboxylic Acid. Molbank, 2024(3), M1871. https://doi.org/10.3390/M1871






