Abstract
We report a straightforward and efficient synthesis of 4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate (3a) and 8-iodo-4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate (3b) in good yields through an O-sulfonylation reaction of 7-hydroxy-2H-chromen-2-ones 1a and 1b with benzenesulfonyl chloride 2 mediated by triethylamine in dichloromethane at ambient temperature. The aryl sulfonyl esters were characterized using spectroscopic, spectrometric, and thermal analyses.
1. Introduction
The versatile properties of coumarins, whether synthetic or natural, have garnered growing interest among researchers in medicinal chemistry and drug design, owing to their broad spectrum of biological and pharmacological activities [1,2,3]. Structurally, coumarin consists of a benzene ring fused to an α-pyrone ring. Among its derivatives known for their intriguing anticancer properties are 7-hydroxycoumarins, with umbelliferone, a natural product, being a prominent member of this family [4,5]. However, there has been limited exploration into functionalizing 7-hydroxycoumarins in recent years [6]. We find this surprising given the high reactivity of the OH group at the C–7 position of coumarins, which is conducive to simple and efficient O-acylation [7], O-alkylation [8], and O-sulfonylation processes [9]. Notably, among these, the O-sulfonylation reaction has received minimal attention.
Therefore, we focused on synthesizing 4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonates 3a and 3b via an O-sulfonylation reaction between 7-hydroxy-4-methyl-2H-chromen-2-one 1a and 1b and benzenesulfonyl chloride 2 in the presence of a base (Scheme 1). Compound 3b has not been reported in the Reaxys database, whereas procedures for synthesizing compound 3a were reported in 1941 and 1958, using sodium ethoxide in ethanol and potassium carbonate in acetone, respectively [10,11]. In 1986, a patent described the synthesis of compound 3a in 81% yield, and its characterization was based solely on elemental analysis [12]. In 2008, compound 3a was synthesized employing an excess of pyridine as both the base and solvent, kept at 5–10 °C with stirring for 30 min, and subsequently analyzed by X-ray crystallography [13]. However, these reports did not include crucial information on spectroscopic, spectrometric, and thermal analyses. Therefore, we devised a novel procedure using a slight excess of triethylamine in dichloromethane at ambient temperature, with stirring for 1 h, resulting in the synthesis of aryl sulfonyl esters 3a and 3b in 77% and 82% yields, respectively (Scheme 1). Furthermore, our investigation offers valuable insights into their spectroscopic, spectrometric, and thermal properties. Remarkably, this research was conducted by three undergraduate students over 16 weeks, dedicating 3 h per week to laboratory work. This opportunity significantly enhanced their comprehension of structural analysis and synthesis techniques as part of two electives in the Chemistry Program at the Universidad Pedagógica y Tecnológica de Colombia.
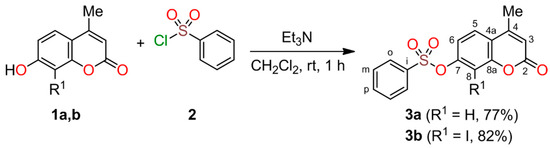
Scheme 1.
Synthesis of aryl sulfonyl esters 3.
2. Results and Discussion
Building on our previous investigations on the functionalization of hydroxy heterocyclic compounds [14,15], we have successfully synthesized 7-hydroxy-4-methyl-2H-chromen-2-one 1a in 72% yield using a previously reported Pechmann condensation protocol [16]. Subsequently, regioselective iodation at the C–8 position of compound 1a produced 7-hydroxy-8-iodo-4-methyl-2H-chromen-2-one 1b in 79% yield [17]. We then developed a straightforward and efficient protocol for synthesizing 4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate 3a by the O-sulfonylation of 7-hydroxy-4-methyl-2H-chromen-2-one 1a with benzenesulfonyl chloride 2 in the presence of triethylamine in dichloromethane at ambient temperature with stirring for 1 h (Scheme 1). After evaporating the dichloromethane under vacuum, the crude material was purified via column chromatography on silica gel using dichloromethane as the eluent to furnish compound 3a in 77% yield. We applied the same procedure to starting material 1b to afford 8-iodo-4-methyl-2-oxo-2H-chromen-7-yl benzenesulfonate 3b in 82% yield. Finally, the structural characterization of aryl sulfonyl esters 3 was carried out using FT-IR, UV–Vis, NMR, mass spectrometry, DSC, and TGA analyses (See Materials and Methods section).
The infrared spectra confirmed the O-sulfonylation of 7-hydroxy-4-methyl-2H-chromen-2-ones 1a and 1b, as evidenced by the disappearance of the O–H stretching vibrations at 3124 and 3150 cm−1, respectively (Figures S5 and S6). The C=O/C–S stretching vibrations of aryl sulfonate esters 3a and 3b were observed at 1734/762 cm−1 and 1728/758 cm−1, respectively. In addition, the asymmetric/symmetric SO2 stretching vibrations of compounds 3a and 3b were assigned at 1365/1192 cm−1 and 1382/1175 cm−1, respectively. The C–I stretching vibration of compound 3b was assigned at 535 cm−1.
The UV–Vis spectra of 7-hydroxy-4-methyl-2H-chromen-2-ones 1 and aryl sulfonate esters 3 were measured in methanol at a concentration of 15 μM within the range of 200 to 400 nm (Figures S9 and S10). The chemical transformation of the –OH into the –OSO2Ph group altered the UV–Vis spectra. Specifically, compounds 3a and 3b showed the appearance of bands at 272 nm (ε = 17,250 L·mol−1·cm−1) and 283 nm (ε = 20,147 L·mol−1·cm−1), respectively, attributed to π → π* transitions within the phenyl ring and the sulfonate ester group. When comparing compounds 3a/3b, we observed a slight hypsochromic shift in the bands at 272/283 nm and 311/318 nm, which can be attributed to an increased band gap caused by the electron-withdrawing effect of the iodine atom.
Table 1 and Table 2 present the assignment of all proton and carbon signals for aryl sulfonate esters 3a and 3b, respectively. The 1H NMR analysis of 3a confirmed the presence of the coumarin ring by identifying a singlet at 6.41 ppm corresponding to the H–3 proton, a doublet of doublets at 7.06 ppm (J = 8.6, 2.2 Hz) assigned to the H-6 proton, and two doublets at 7.12 ppm (J = 2.4 Hz) and 7.78 ppm (d, J = 8.4 Hz) corresponding to the H–8 and H–5 protons, respectively. In contrast, the 1H NMR analysis of 3b did not show the ABX system due to an iodine atom at the eighth position of the coumarin ring. The absence of the OH proton indicates the successful O-sulfonylation process. At the same time, the introduction of the –OSO2Ph substituent in compound 3a was confirmed by a triplet of triplets at 7.85 ppm (J = 7.4, 1.2 Hz) and two doublets of doublets at 7.69 ppm (J = 8.0, 8.0 Hz) and 7.92 ppm (J = 8.4, 1.2 Hz).

Table 1.
NMR assignments and correlations of compound 3a.

Table 2.
NMR assignments and correlations of compound 3b.
Similarly, compound 3b exhibited a triplet at 7.88 ppm (J = 7.4 Hz), a doublet of doublets at 7.71 ppm (J = 7.8, 7.8 Hz), and a doublet at 7.95 ppm (J = 7.6 Hz). The 13C-NMR and DEPT-135 analysis of aryl sulfonate ester 3a revealed one methyl carbon (18.1 ppm), seven aromatic methines (110.3, 114.5, 118.1, 127.1, 128.3, 130.0, and 135.4 ppm), and six quaternary aromatic carbons (118.9, 133.9, 150.6, 152.6, 153.3, and 159.2 ppm). Similarly, the 13C-NMR and DEPT-135 analysis of 3b displayed one methyl carbon, six aromatic methines, and seven quaternary aromatic carbons. The presence of the iodine atom in the coumarin ring of 3b caused higher shielding of the C–8 carbon (Cq, 84.0 ppm) compared to the C–8 carbon in the iodine-free coumarin 3a (CH, 110.3 ppm). The analysis of 2D NMR spectra, including HSQC, HMBC, and COSY, allowed for an unambiguous structural assignment.
Principio del formulario The exact masses of the pseudo-molecular ions ([M + H]+) and their corresponding elemental formulas, C16H13O5S+ and C16H12IO5S+, were confirmed through high-resolution mass spectrometry, affording mass errors of 2.84 and 0.22 ppm, respectively (Figures S3 and S4).
Aryl sulfonate esters 3a and 3b underwent separate TGA and DSC analyses, as shown in Figures S26 and S27, respectively. The thermal analysis was conducted under a nitrogen atmosphere, spanning temperatures from 25 to 400 °C, maintaining a constant gas flow of 25 mL min−1 and a heating rate of 10 °C min−1. The TGA thermograms of compounds 3a and 3b indicate high thermal stability, as evidenced by the temperature ranges of 274 to 329 °C and 282 to 328 °C, respectively. In addition, the DSC thermograms show endothermic peaks at 115 and 200 °C for compounds 3a and 3b, respectively, suggesting that the melting process involves moderate heat absorption of 89.8 and 78.0 J g−1. Conversely, the exothermic peaks at 350 and 308 °C, accompanied by substantial heat emissions of 173.5 and 147.5 J g−1, likely indicate structural transformations within compounds 3a and 3b, respectively.
3. Conclusions
In summary, we developed a novel protocol for synthesizing aryl sulfonyl esters 3a and 3b with good yields under mild-reaction conditions. Our protocol offers several advantages, including reduced reaction time, operational simplicity, and the use of a slight excess of triethylamine, which can be easily removed under reduced pressure, eliminating the need for an additional extraction process. Additionally, we conducted an extensive characterization of the aryl sulfonyl esters using spectroscopic, spectrometric, and thermal analyses.
4. Materials and Methods
4.1. General Information
The progression of reactions was monitored using Thin Layer Chromatography employing a UV lamp with wavelengths of 254 or 365 nm. Flash column chromatography was performed using silica gel 60 (mesh 230–400) (Alfa Aesar, Tewksbury, MA, USA). An ATR accessory-equipped PerkinElmer FT-IR spectrometer (PerkinElmer, Inc., Waltham, MA, USA) was employed to acquire infrared spectra at room temperature. UV–Vis spectra were obtained from a methanol solution (1.5 × 10−5 M) using a UV–Vis Evolution 201 spectrophotometer (Thermo Fischer Scientific Inc., Madison, WI, USA). The Bruker Advance 400 spectrophotometer (Bruker BioSpin GmbH, Rheinstetten, Germany) was utilized for acquiring NMR spectra in DMSO-d6. Chemical shifts are reported in ppm (δ), and coupling constants are expressed in Hz (J). The 1H and 13C NMR spectra were calibrated using the residual non-deuterated signal (δ = 2.50 ppm) and the deuterated solvent signal (δ = 39.52 ppm), respectively. High-resolution mass spectrometry (HRMS) was conducted using a Q-TOF 6520 spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) with electrospray ionization (ESI, 4000 V). Measurements for both differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were independently performed utilizing the STA7200 thermogravimetry/differential thermal analyzer (Hitachi America Ltd., Santa Clara, CA, USA).
4.2. Synthesis of 7-Hydroxy-4-methyl-2H-chromen-2-one (1a)
Concentrated H2SO4 (5.5 mL, 30.0 mmol) was placed under magnetic stirring in an ice bath to maintain the temperature at approximately 0 °C. Meanwhile, a separate solution containing resorcinol (1101 mg, 10.0 mmol) in methyl acetoacetate (1.08 mL, 10.0 mmol) was prepared. This solution was then slowly added drop by drop to the concentrated H2SO4 while ensuring the temperature remained at approximately 0 °C and continuous stirring. After completing the addition, stirring continued for 30 min at room temperature. Subsequently, the mixture was poured over crushed ice (approximately 50 g) to afford a solid product. The solid product was dissolved by adding an aqueous solution of NaOH (25.0 mL, 1.0 M), followed by the addition of an HCl solution (6.0 M) drop by drop until the pH reached 2.0. The precipitate was filtered, washed with distilled water (5.0 mL), and purified by recrystallization using ethanol (10.0 mL). Subsequently, the precipitate was filtered, washed with cold ethanol (5.0 mL), and dried to yield compound 1a as a brown solid (1268 mg, 72%). M.p. 186–187 °C (Lit. 187–189 °C) [3]. FTIR–ATR: ν = 3124 (ν O–H), 3011, 2886, 2805, 1673 (ν C=O), (1591 and 1571, ν C=C), 1391, 1272, (1237 and 1212, ν C–O–C), 1067 (ν C–O–C), 981, 844, 745, 693, 641, 583 cm−1. UV–Vis (Methanol, 15 μM) λmax (ε, L·mol−1·cm−1): 202 (47,449, π → π*), 216 (16,417, π → π*), 322 (14,559, π → π* and n → π*) nm. 1H NMR (400 MHz, DMSO-d6) δ = 2.35 (s, 3H), 6.11 (s, 1H), 6.70 (d, J = 1.2 Hz, 1H), 6.79 (dd, J = 8.8, 2.0 Hz, 1H), 7.57 (dd, J = 8.8, 2.4 Hz, 1H), 10.52 (br s, 1H, OH) ppm. 13C{1H} NMR (101 MHz, DMSO-d6): δ = 18.1 (CH3), 102.2 (CH), 110.3 (CH), 112.0 (Cq), 112.9 (CH), 126.6 (CH), 153.5 (Cq), 154.8 (Cq), 160.3 (Cq), 161.2 (Cq) ppm. HRMS (ESI+): calcd for C10H9O3+, 177.0546 [M + H]+; found, 177.0543. These NMR data match previously reported data [3].
4.3. Synthesis of 7-Hydroxy-8-iodo-4-methyl-2H-chromen-2-one (1b)
7-Hydroxy-4-methyl-2H-chromen-2-one 1a (440 mg, 2.50 mmol) was dissolved in 2.0 mL of aqueous ammonium hydroxide (5.3 M). A solution of iodine (634 mg, 2.50 mmol) in 20.0 mL of aqueous potassium iodide (0.32 M) was then added dropwise. The reaction mixture was stirred at room temperature for 2 h. Then, aqueous hydrochloric acid (2.9 M) was added dropwise until the pH reached 2.0. The resulting precipitate was filtered, washed with distilled water (5.0 mL), and purified by recrystallization from ethanol (10.0 mL). Subsequently, the precipitate was filtered again, washed with cold ethanol (5.0 mL), and dried to yield compound 1b as a yellow solid (596 mg, 79%). M.p. 231–233 °C (Lit. 234–236 °C) [17]. FTIR–ATR: ν = 3150 (ν O–H), 3011, 2886, 2805, 1682 (ν C=O), 1598 (ν C=C), 1381, 1308, (1247 and 1226, ν C–O–C), (1079 and 1045, ν C–O–C), 1004, 908, 851, 809, 756, 702, 600, 538 (ν C–I) cm−1. UV–Vis (Methanol, 15 μM) λmax (ε, L·mol−1·cm−1): 204 (59,237, π → π*), 265 (9543, π → π*), 322 (20,711, π → π* and n → π*) nm. 1H NMR (400 MHz, DMSO-d6) δ = 2.37 (s, 3H), 6.18 (s, 1H), 6.91 (d, J = 8.8 Hz, 1H), 7.60 (d, J = 8.4 Hz, 1H), 11.36 (br s, 1H, OH) ppm. 13C{1H} NMR (101 MHz, DMSO-d6): δ = 18.2 (CH3), 74.6 (Cq), 110.7 (CH), 111.6 (CH), 112.8 (Cq), 126.4 (CH), 153.6 (Cq), 154.3 (Cq), 160.0 (Cq), 161.0 (Cq) ppm. HRMS (ESI+): calcd for C10H8IO3+, 302.9513 [M + H]+; found, 302.9508. These NMR data match previously reported data [17].
4.4. Synthesis of 4-Methyl-2-oxo-2H-chromen-7-yl Benzenesulfonate (3a)
A mixture of 7-hydroxy-4-methyl-2H-chromen-2-one 1a (100 mg, 0.57 mmol), benzenesulfonyl chloride 2 (87 µL, 0.68 mmol), and triethylamine (95 µL, 0.68 mmol) in dichloromethane (2.0 mL) was stirred at room temperature for 1 h. Subsequently, the solvent was removed under vacuum pressure, and the solid was purified by flash column chromatography on silica gel using dichloromethane as the mobile phase to yield compound 3a as a colorless solid (139 mg, 77%). M.p. 113–114 °C (Lit. 104–105 °C and 107–108 °C) [10,11]. FTIR–ATR: ν = 3106, 3078, 2882, 2812, 1734 (ν C=O), (1627 and 1609, ν C=C), 1492, 1447, 1365 (νa SO2), 1259 (ν C–O–C), 1192 (νs SO2), 1175, 1150, 1118, 1089, 1065 (ν C–O–C), 978, 887, 862, 837, 762 (ν C–S), 687, 631, 582, 567, 529 cm−1. UV–Vis (Methanol, 15 μM) λmax (ε, L·mol−1·cm−1): 202 (56,732, π → π*), 211 (42,543, π → π*), 272 (17,250, π → π*), 311 (12,822, π → π* and n → π*) nm. 1H NMR (400 MHz, DMSO-d6) δ = 2.39 (s, 3H, CH3), 6.41 (s, 1H, H–3), 7.06 (dd, J = 8.6, 2.2 Hz, 1H, H–6), 7.12 (d, J = 2.4 Hz, 1H, H–8), 7.69 (dd, J = 8.0, 8.0 Hz, 2H, Hm), 7.78 (d, J = 8.4 Hz, 1H, H–5), 7.85 (tt, J = 7.4, 1.2 Hz, 1H, Hp), 7.92 (dd, J = 8.4, 1.2 Hz, 2H, Ho) ppm. 13C{1H} NMR (101 MHz, DMSO-d6): δ = 18.1 (CH3), 110.3 (CH, C–8), 114.5 (CH, C–3), 118.1 (CH, C–6), 118.9 (Cq, C–4a), 127.1 (CH, C–5), 128.3 (2CH, Co), 130.0 (2CH, Cm), 133.9 (Cq, Ci), 135.4 (CH, Cp), 150.6 (Cq, C–7), 152.6 (Cq, C–4), 153.3 (Cq, C–8a), 159.2 (Cq, C–2) ppm. HRMS (ESI+): calcd for C16H13O5S+, 317.0478 [M + H]+; found, 317.0487.
4.5. Synthesis of 8-Iodo-4-methyl-2-oxo-2H-chromen-7-yl Benzenesulfonate (3b)
A mixture of 7-hydroxy-8-iodo-4-methyl-2H-chromen-2-one 1b (172 mg, 0.57 mmol), benzenesulfonyl chloride 2 (87 µL, 0.68 mmol), and triethylamine (95 µL, 0.68 mmol) in dichloromethane (2.0 mL) was stirred at room temperature for 1 h. Subsequently, the solvent was removed under vacuum pressure, and the solid was purified by flash column chromatography on silica gel using dichloromethane as the mobile phase to yield compound 3b as a colorless solid (207 mg, 82%). FTIR–ATR: ν = 3069, 2982, 1728 (ν C=O), (1621 and 1589, ν C=C), 1447, 1382 (νa SO2), 1261 (ν C–O–C), 1175 (νs SO2), 1079 (ν C–O–C), 1026, 1003, 862, 820, 758 (ν C–S), 734, 683, 627, 576, 535 (ν C–I) cm−1. UV–Vis (Methanol, 15 μM) λmax (ε, L·mol−1·cm−1): 202 (61,895, π → π*), 283 (20,147, π → π*), 318 (9637, π → π* and n → π*) nm. 1H NMR (400 MHz, DMSO-d6) δ = 2.42 (s, 3H, CH3), 6.45 (s, 1H, H–3), 7.16 (d, J = 8.8 Hz, 1H, H–6), 7.71 (dd, J = 7.8, 7.8 Hz, 2H, Hm), 7.84 (d, J = 8.8 Hz, 1H, H–5), 7.88 (t, J = 7.4 Hz, 1H, Hp), 7.95 (d, J = 7.6 Hz, 2H, Ho) ppm. 13C{1H} NMR (101 MHz, DMSO-d6): δ = 18.2 (CH3), 84.0 (Cq, C–8), 114.8 (CH, C–3), 117.9 (CH, C–6), 119.1 (Cq, C–4a), 126.8 (CH, C–5), 128.4 (2CH, Co), 130.1 (2CH, Cm), 134.7 (Cq, Ci), 135.6 (CH, Cp), 152.0 (Cq, C–7), 152.7 (Cq, C–4), 153.8 (Cq, C–8a), 159.2 (Cq, C–2) ppm. HRMS (ESI+): calcd for C16H12IO5S+, 442.9445 [M + H]+; found, 442.9446.
Supplementary Materials
The following are available online: Figure S1: HRMS spectrum of the compound 1a, Figure S2: HRMS spectrum of the compound 1b, Figure S3: HRMS spectrum of the compound 3a, Figure S4: HRMS spectrum of the compound 3b, Figure S5: FT-IR spectrum of the compound 1a (ATR Technique), Figure S6: FT-IR spectrum of the compound 1b (ATR Technique), Figure S7: FT-IR spectrum of the compound 3a (ATR Technique), Figure S8: FT-IR spectrum of the compound 3b (ATR Technique), Figure S9: UV–Vis spectra for compounds 1a and 3a, Figure S10: UV–Vis spectra for compounds 1b and 3b, Figure S11: 1H NMR spectrum of the compound 1a, Figure S12: 13C{1H} NMR and DEPT-135 spectra of the compound 1a, Figure S13: COSY spectrum of the compound 1a, Figure S14: 1H NMR spectrum of the compound 1b, Figure S15: 13C{1H} NMR and DEPT-135 spectra of the compound 1b, Figure S16: 1H NMR spectrum of the compound 3a, Figure S17: 13C{1H} NMR and DEPT-135 spectra of the compound 3a, Figure S18: HSQC spectrum of the compound 3a, Figure S19: HMBC spectrum of the compound 3a, Figure S20: COSY spectrum of the compound 3a, Figure S21: 1H NMR spectrum of the compound 3b, Figure S22: 13C{1H} NMR and DEPT-135 spectra of the compound 3b, Figure S23: HSQC spectrum of the compound 3b, Figure S24: HMBC spectrum of the compound 3b, Figure S25: COSY spectrum of the compound 3b, Figure S26: TGA and DSC thermograms of compound 3a, and Figure S27: TGA and DSC thermograms of compound 3b.
Author Contributions
Investigation and data curation, L.P.-M.; investigation and data curation, A.P.-T.; investigation and data curation, A.L.-B.; investigation and data curation, M.A.M.; investigation, data curation, and writing—original draft preparation, D.B.; conceptualization, investigation, data curation, and writing—original draft preparation, J.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors are thankful for financial support from the Escuela de Ciencias Químicas at the Universidad Pedagógica y Tecnológica de Colombia. M.A.M. acknowledges support from the Department of Chemistry at the Universidad de Los Andes. Additionally, we also acknowledge Universidad de Alcalá for acquiring NMR spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Somappa, S.B. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure–activity relationship. J. Clin. Pharm. Ther. 2022, 47, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic effects of coumarins with different substitution patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Yu, S.-M.; Hu, D.-H.; Zhang, J.-J. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 3869–3873. [Google Scholar] [CrossRef]
- Kandil, S.; Westwell, A.D.; McGuigan, C. 7-Substituted umbelliferone derivatives as androgen receptor antagonists for the potential treatment of prostate and breast cancer. Bioorg. Med. Chem. Lett. 2016, 26, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; González-Marrero, J.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, A.G.T.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, M.-L.; Yuan, M.-S. Antifeedant activities of tutin and 7-hydroxycoumarin acylation derivatives against Mythimna separate. J. Pestic. Sci. 2012, 37, 95–98. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, S.S.; Salmas, R.E.; Durdagi, S.; Epifano, F.; Genovese, S.; Fiorito, S. Combined molecular modeling and cholinesterase inhibition studies on some natural and semisynthetic O-alkylcoumarin derivatives. Bioorg. Chem. 2019, 84, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, R.O.; Pentikäinen, O.; Huuskonen, J.; Timonen, J.; Kärkkäinen, O.; Heikkinen, A.; Fashe, M.; Raunio, H. In vitro sulfonation of 7-hydroxycoumarin derivatives in liver cytosol of human and six animal species. Xenobiotica 2020, 50, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Schamschurin, I. <NS> Nr. 25 Chimija Nr. 1 <1941> 1, 5. Chem. Abstr. 1941, 5876. [Google Scholar]
- Esajan, W. Chem. Abstr. 1958, 12854.
- Sulfonates of Hydroxycoumarins. Current Patent Assignee: BASF SE—US4618622, 21 October 1986.
- Yang, S.-P.; Wang, D.-Q.; Hanc, L.-J.; Liu, Y.-F. 4-Methyl-2-oxo-2,3-dihydro-1-benzopyran-7-yl benzenesulfonate. Acta Cryst. 2008, E64, o2088. [Google Scholar] [CrossRef] [PubMed]
- Becerra, D.; Rojas, H.; Castillo, J.-C. Synthesis, spectroscopic, and thermal analyses of 2-oxo-1,2-dihydroquinolin-8-yl 4-chlorobenzoate. Molbank 2023, 2023, M1672. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Becerra, D.; Macías, M.A. Crystal structure, hirshfeld surface analysis, and computational study of quinolin-8-yl 4-chlorobenzoate: Insights from spectroscopic, thermal, and antitumor properties. Crystals 2023, 13, 694. [Google Scholar] [CrossRef]
- Timonen, J.M.; Nieminen, R.M.; Sareila, O.; Goulas, A.; Moilanen, L.J.; Haukka, M.; Vainiotalo, P.; Moilanen, E.; Aulaskari, P.H. Synthesis and anti-inflammatory effects of a series of novel 7-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2011, 46, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Gavinolla, V.; Thangalipalli, S.; Bandalla, S.G.; Panduga, R.; Neella, C.K. A thermo-regulated highly regioselective mono and dihalogenations of phenols and anilines in water employing new Lewis base adducts (LBAs) [DBUBr]+Br− and [DBUI]+I− as green reagents: A simple approach. New J. Chem. 2023, 47, 20777–20784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).