Abstract
(1R,3R,5S,Z)-2-ethylidene-6,6-dimethyl-3-vinylbicyclo[3.1.1]heptane was prepared by hydrovinylation of nopadiene catalyzed by a cationic Ru complex. The structure was fully characterized by 1H- and 13C-NMR spectroscopy, including 2D-COSY and 2D-NOESY spectra, optical rotation, and combustion analysis. In contrast to the previously reported 1,2-hydrovinylation of 1-vinylcycloalkenes by this catalyst, the reaction with nopadiene proceeds by 1,4-addition of ethylene.
1. Introduction
Transition metal catalyzed hydrovinylation is an atom economical C–C bond forming reaction [1,2], which has seen increasing utility in natural product synthesis [3,4]. Complexes of nickel [4,5,6], cobalt [6,7,8,9,10], iron [11], palladium [12], and ruthenium [13,14,15,16,17] have been reported as catalysts for this reaction. In general, for 1-vinylcycloalkene substrates Ni- and Ru-based catalysts proceed with 1,2-selectivity (Scheme 1). In contrast, Co-catalysis proceeds with 1,4-regioselectivity, with the cis-stereoisomer predominating. We herein report that the hydrovinylation of nopadiene (1), utilizing cationic Ru-complex 2, proceeds with unanticipated 1,4-regioselectivity with trans-stereoselectivity.
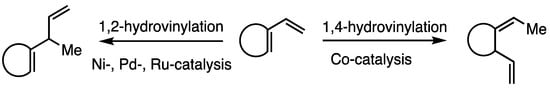
Scheme 1.
Hydrovinylation of 1-vinylcycloalkenes.
2. Results
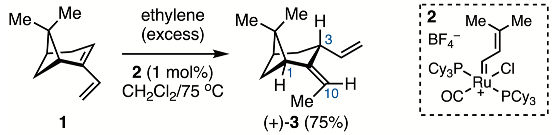
The reaction of nopadiene (1) with excess ethylene, catalyzed by cationic Ru-complex 2, produced a mixture of a hydrovinylation product (3) along with unreacted 1 (Equation (1)). To separate the two hydrocarbons, the mixture was titrated with a solution of 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), which reacted rapidly with nopadiene via a hetero-Diels–Alder reaction, until the red color of PTAD persisted. Chromatographic purification of this mixture gave 3.
The structure of 3 was assigned based on its 1H NMR spectral data (see Supplementary Materials). In particular, the signals at δ 5.24 (dq, J = 1.5, 6.9 Hz, 1H), 3.04 (br t, J = 8.0 Hz, 1H), 2.87 (t, J = 5.7 Hz, 1H), and 1.57 (dd, J = 1.5, 6.9 Hz, 3H) were assigned to H(10), H(3), H(1), and the allylic methyl group. The absence of any 3JH-H coupling between the diallylic proton H(3) and any of the methyl signals excluded a 1,2-hydrovinylation structure. A small cross-peak in the NOESY NMR spectrum of 3 was observed between the allylic methyl group and H(1), suggesting a (Z)-stereochemical assignment. However, this stereochemical assignment must be considered tentative, as a cross-peak on the opposite side of the diagonal could not be observed, in spite of extensive attempts at manual phase correction along F2. Notably, no cross-peak between H(10) and H(1) was evident. The vinyl group at C(3) was tentatively assigned an orientation opposite the gem-dimethyls of the bicyclo[3.1.1]heptane system. These tentative assignments were eventually corroborated by X-ray diffraction analysis of a crystalline derivative [18].
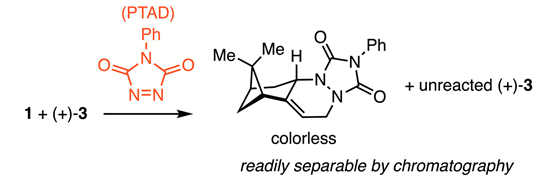
3. Discussion
The hydrovinylation of 1 is anticipated to involve coordination of the less substituted olefin of nopadiene to a ruthenium–hydride species (i.e., 4, Scheme 2). Reversible insertion of the coordinated olefin into the Ru–H bond generates the π-allyl-Ru intermediate 5, which has the allylic methyl group in a syn-orientation. Notably, the π-allyl-Pd complex generated by the reaction of ethylidenenorpinane with PdCl2 was assigned a similar structure based on its 1H NMR spectral data, as well as an X-ray crystal structure [19]. Coordination of ethylene to 5 affords a σ-allyl intermediate 6. Insertion of the coordinated ethylene occurs with retention of the configuration to generate 7. The product that (+)-3 produced by β-hydride elimination from 7 resulted in ligand exchange. The formation of the 1,4-addition product, in contrast to the 1,2-addition product which we have observed for all other 1-vinylcycloalkenes [13], may be rationalized on the lower energy of σ-allyl intermediate 6 compared to 8. Intermediate 6 should be lower in energy due to the additional strain in 8 from the C2–C3 olefin within the strained bicyclo[3.1.1]heptane ring system.
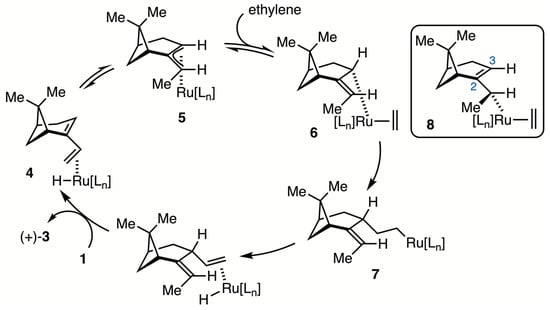
Scheme 2.
Proposed catalytic cycle for hydrovinylation catalyzed by Ru-complex.
4. Materials and Methods
4.1. General
All 1H NMR and 13C NMR spectra were recorded using a Varian 300 MHz spectrometer in CDCl3. 1H spectra were calibrated according to the residual signal of CHCl3 (δ 7.26 ppm) and 13C spectra were calibrated to the central peak for CDCl3 (δ 77.0 ppm). Optical rotations were recorded on a Perkin Elmer 341 optical polarimeter at a 589 nm wavelength (20 °C). Elemental analysis was obtained from Midwest Microlabs, Ltd. (Indianapolis, IN, USA). Nopadiene (1) [20] and catalyst 2 [21] were prepared following the literature procedures.
4.2. Synthesis of (1R,3R,5S,Z)-2-Ethylidene-6,6-dimethyl-3-vinylbicyclo[3.1.1]heptane (3)
Inside a nitrogen-filled glovebox, a 25 mL medium-walled vacuum Schlenk tube equipped with stirring bar and Teflon stopcock was charged with nopadiene (0.2 mL, 1.07 mmol), cationic catalyst 2 (1.0 mol%), and methylene chloride (3 mL). The tube was removed from the glove box, cooled in a liquid N2 bath, and excess ethylene (ca. 6.4 mmol) was condensed into the tube. The tube was stoppered, removed from the liquid N2 bath, warmed to room temperature, and immersed in an oil bath at 75 °C for 15 h [NOTE: an explosion safety shield should be used between the heating reaction mixture and the fume hood sash]. After this time, the reaction mixture was cooled to room temperature and the tube opened to the air. The reaction mixture was concentrated, and the residue was dissolved in hexanes/dichloromethane and passed through a short column of silica gel in a disposable pipet to remove the catalyst. The crude product was dissolved in CH2Cl2 and small amounts of a solution of 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) in CH2Cl2 were added to the stirred solution until the red color of the PTAD persisted. The mixture was concentrated and the residue purified by column chromatography (SiO2, hexanes) to afford 3 as a colorless oil (0.142 g, 75%): [α]D20 = +4.70 (c = 4.4, CH2Cl2). 1H NMR (300 MHz, CDCl3) δ = 5.85 (ddd, J = 8.0, 9.9, 16.8 Hz, 1H), 5.24 (dq, J = 1.5, 6.9 Hz, 1H), 4.92 (ddd, J = 1.5, 2.1, 16.8 Hz, 1H), 4.85 (ddd, J = 1.0, 2.1, 9.9 Hz, 1H), 3.04 (br t, J = 8.0 Hz, 1H), 2.87 (t, J = 5.7 Hz, 1H), 2.33 (dtd, J = 2.1, 6.0, 9.9 Hz, 1H), 2.22 (tdd, J = 2.1, 10.2, 13.8 Hz, 1H), 2.05–1.96 (m, 1H), 1.72 (ddd, J = 2.7, 3.9, 13.8 Hz, 1H), 1.57 (dd & m, J = 1.5, 6.9 Hz, 4H), 1.29 (s, 3H), 0.78 (s, 3H). 13C NMR (75 MHz, CDCl3) δ = 146.8 (CH), 1.42 (C), 118.5 (CH), 110.9 (CH2), 44.4 (CH), 41.4 (CH), 40.8 (C), 40.7 (CH), 32.0 (CH2), 28.2 (CH2), 26.6 (CH3), 22.1 (CH3), 13.1 (CH3). Anal. calcd for C13H20 (176.30): C, 88.57; H, 11.43. Found: C, 88.63; H, 11.47.
Supplementary Materials
1H NMR spectrum of 3 (δ 0–10 ppm); 1H NMR spectrum of 3 (expansion of region δ 1.6–6.0 ppm); 2D-COSY 1H NMR spectrum of 3; 2D-NOESY 1H NMR spectrum of 3; 13C NMR spectrum of 3.
Author Contributions
Conceptualization, W.A.D.; methodology, Z.H.; validation, Z.H.; investigation, Z.H.; resources, W.A.D.; data curation, W.A.D.; writing—original draft preparation, W.A.D.; writing—review and editing, Z.H. and W.A.D.; supervision, W.A.D.; project administration, W.A.D.; funding acquisition, W.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, grant number GM42641.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to legal reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goossen, L.J. Asymmetric Hydrovinylation: New Perspectives through Use of Modular Ligand Systems. Angew. Chem. Int. Ed. 2002, 41, 3775–3778. [Google Scholar] [CrossRef]
- RajanBabu, T.V. Asymmetric Hydrovinylation Reaction. Chem. Rev. 2003, 103, 2845–2860. [Google Scholar] [CrossRef] [PubMed]
- RajanBabu, T.V.; Cox, G.A.; Lim, H.J.; Nomura, N.; Sharma, R.K.; Smith, C.R.; Zhang, A.; Mans, D.J. Hydrovinylation Reactions in Organic Synthesis. In Comprehensive Organic Synthesis II.; Molander, G.A., Knochel, P., Eds.; Elsevier: Oxford, UK, 2014; Volume 5, pp. 1582–1620. [Google Scholar]
- Mans, D.J.; Cox, G.A.; RajanBabu, T.V. Ethylene in Organic Synthesis. Repetitive Hydrovinylation of Alkenes for Highly Enantioselective Syntheses of Pseudopterosins. J. Am. Chem. Soc. 2011, 133, 5776–5779. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Leitner, W. Nickel-catalysed enantioselective hydrovinylation of styrenes in liquid or supercritical carbon dioxide. J. Chem. Soc. Chem. Commun. 1999, 1583–1584. [Google Scholar] [CrossRef]
- Zhang, A.; RajanBabu, T.V. Hydrovinylation of 1,3-Dienes: A New Protocol, and Asymmetric Variation, and a Potential Solution to the Exocyclic Side Chain Stereochemistry Problem. J. Am. Chem. Soc. 2006, 128, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Page, J.P.; RajanBabu, T.V. Asymmetric Hydrovinylation of 1-Vinylcycloalkenes. Reagent Control of Regio-and Stereoselectivity. J. Am. Chem. Soc. 2012, 134, 6556–6559. [Google Scholar] [CrossRef] [PubMed]
- Hilt, G.; du Mesnil, F.-X.; Luers, S. An Efficient Cobalt(I) Catalyst System for the Selective 1,4-Hydrovinylation of 1,3-Dienes. Angew. Chem. Int. Ed. 2001, 40, 387–389. [Google Scholar] [CrossRef]
- Arndt, M.; Dindaroglu, M.; Schmalz, H.-G.; Hilt, G. Gaining Absolute Control of the Regiochemistry in Cobalt-Catalyzed 1,4-Hydrovinylation Reaction. Org. Lett. 2011, 13, 6236–6239. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Dindaroglu, M.; Schmalz, H.-G.; Hilt, G. Ligand Control of the Cobalt-Catalyzed 1,4-Hydrovinylation Reaction. Synthesis 2012, 44, 3534–3542. [Google Scholar]
- Moreau, B.; Wu, J.Y.; Ritter, T. Iron-Catalyzed 1,4-Addition of α-Olefins to Dienes. Org. Lett. 2009, 11, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; O’Dair, M.; Sigman, M.S. Synthesis of Highly Functionalized Tri- and Tetrasubstituted Alkenes via Pd-Catalyzed 1,2-Hydrovinylation of Terminal 1,3-Dienes. J. Am. Chem. Soc. 2015, 137, 608–611. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yi, C.S.; Donaldson, W.A. Ruthenium-Catalyzed Hydrovinylation of 1,3-Dienes: Application to the Generation of a 20(S) Steroidal Side Chain. Org. Lett. 2003, 5, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- RajanBabu, T.V.; Nomura, N.; Jin, J.; Nandi, M.; Park, H.; Sun, X. Heterodimerization of Olefins. 1. Hydrovinylation Reactions of Olefins That Are Amenable to Asymmetric Catalysis. J. Org. Chem. 2003, 68, 8431–8446. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yi, C.S.; Donaldson, W.A. Ruthenium-Catalyzed Hydrovinylation of Dienoates: Model Studies Directed Toward the C10-C18 Segment of Ambruticin. Synlett 2004, 1312–1314. [Google Scholar] [CrossRef]
- Sanchez, R.P., Jr.; Connell, B.T. A Ruthenium-Based Catalyst System for Hydrovinylation at Room Temperature. Organometallics 2008, 27, 2902–2904. [Google Scholar] [CrossRef]
- Jiang, G.; List, B. Enantioselective hydrovinylation via asymmetric counteranion-directed ruthenium catalysis. Chem. Commun. 2011, 47, 10022–10024. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Lindeman, S.; Donaldson, W.A. (E)-1-(2,4-dinitrophenyl)-2-(2-((1R,3R,5S,Z)-2-ethylidene-6,6-dimethylbicyclo[3.1.1]heptan-3-yl)ethylidene)hydrazine. Molbank. submission.
- Trost, B.M.; Strege, P.E.; Weber, L.; Fullerton, T.J.; Dietsche, T.J. Allylic alkylation: Preparation of p-allylpalladium complexes from olefins. J. Am. Chem. Soc. 1978, 100, 3407–3415. [Google Scholar] [CrossRef]
- Wu, J.Y.; Moreau, B.; Ritter, T. Iron-Catalyzed 1,4-Hydroboration of 1,3-Dienes. J. Am. Chem. Soc. 2009, 131, 12915–12917. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.S.; Lee, D.W.; Chen, Y. Hydrovinylation and [2+2] Cycloaddition Reactions of Alkynes and Alkenes Catalyzed by a Well-Defined Cationic Ruthenium–Alkylidene Complex. Organometallics 1999, 18, 2043–2045. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).