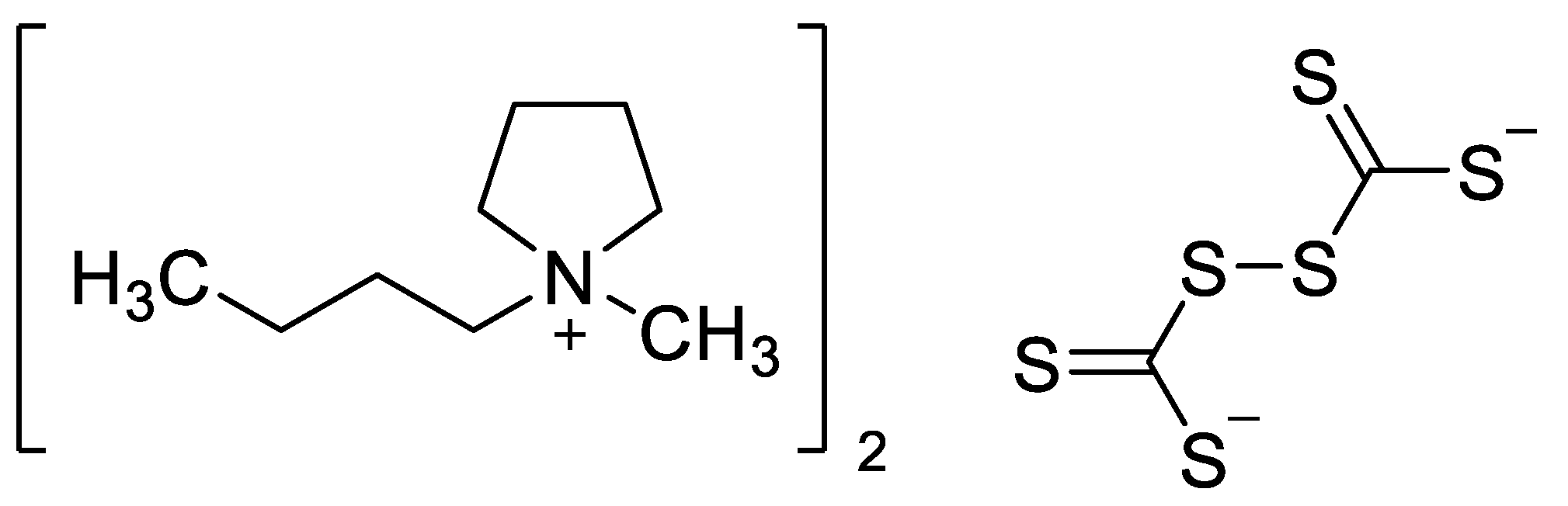
Crystal Structure of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate Complex
Abstract
1. Introduction
2. Results and Discussion
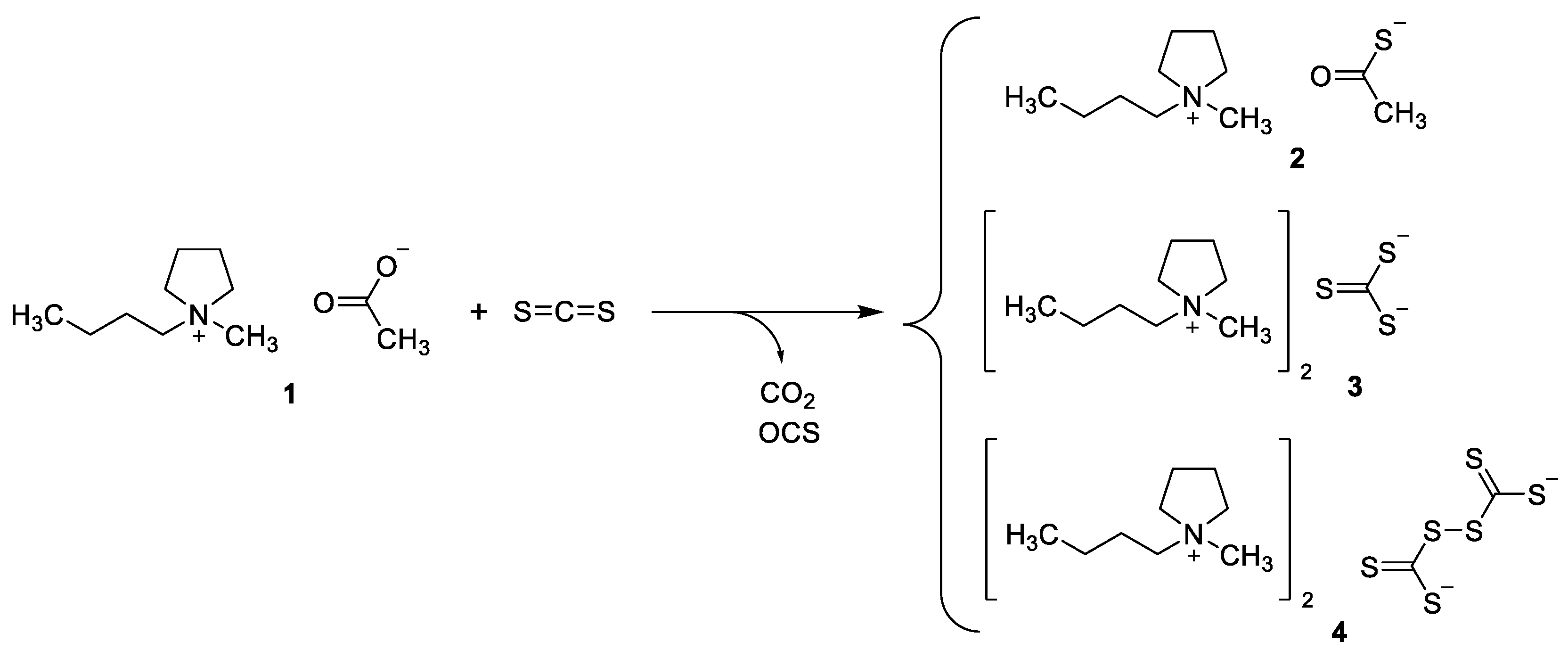
2.1. Synthesis of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate 4
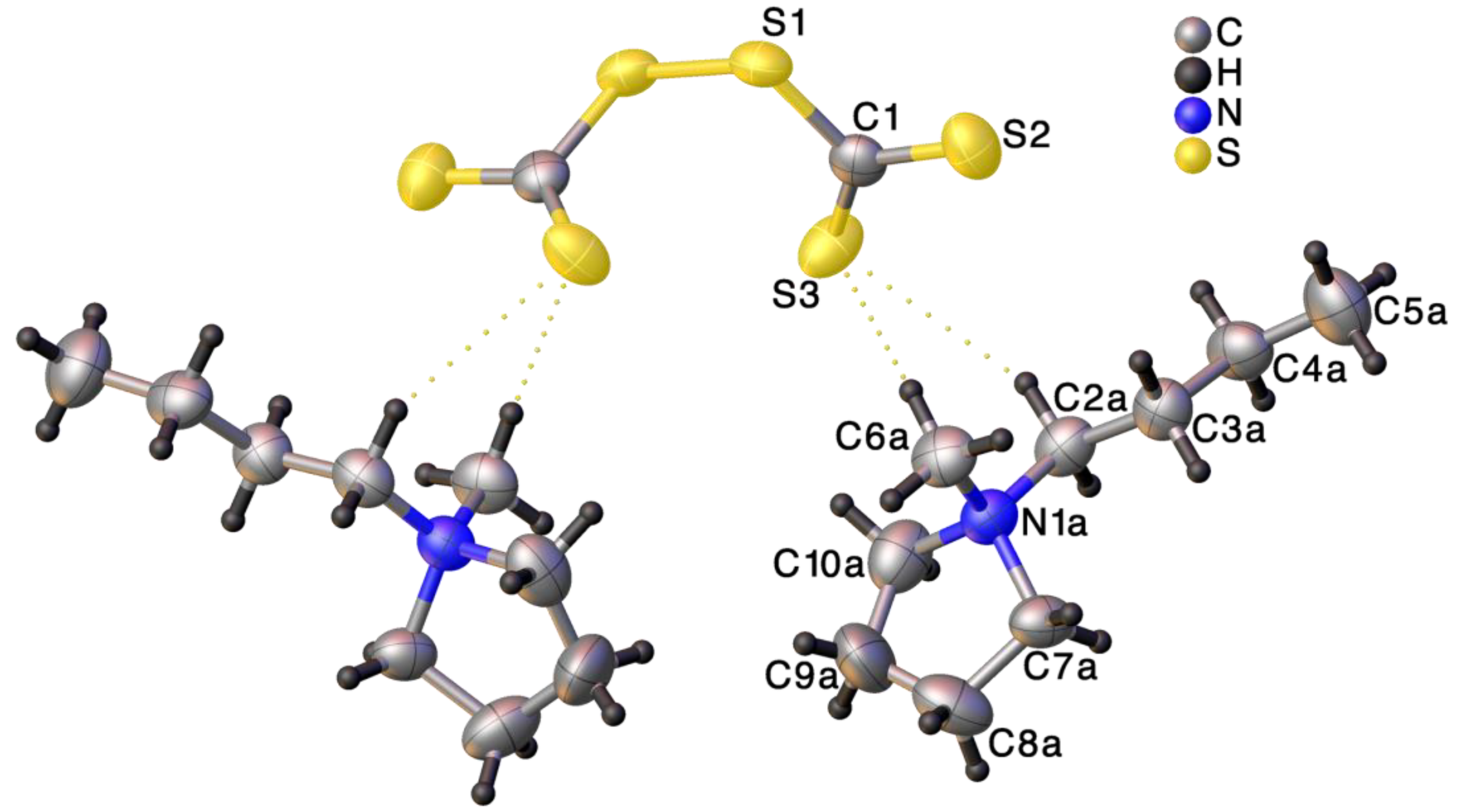
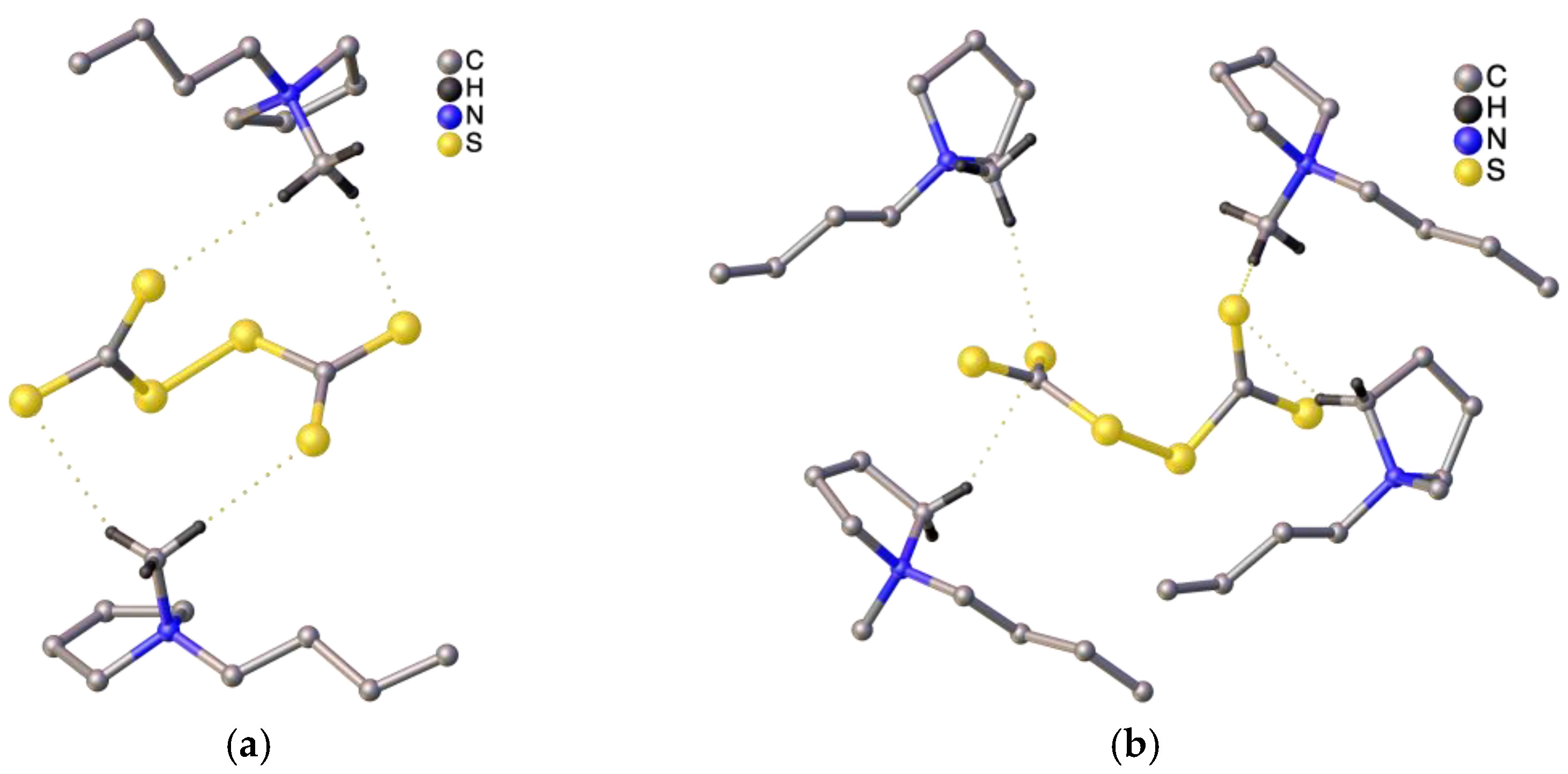
2.2. Crystallographic Structure
3. Materials and Methods
3.1. Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate
3.2. Crystal and Refinement Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Bernhardt, P.; Simone, M. Structural characterization of new ionic liquids via X-ray crystallography. J. Mol. Liq. 2020, 319, 114091. [Google Scholar] [CrossRef]
- Cabaço, I.; Besnard, M.; Vaca Chavez, F.; Pinaud, N.; Sebastiao, P.J.; Coutinho, J.A.P.; Danten, Y. Understanding chemical reactions of CO2 and its isoelectronic molecules with 1-butyl-3-methylimidazolium acetate by changing the nature of the cation: The case of CS2 in 1-butyl-1-methylpyrrolidinium acetate studied by NMR spectroscopy and density functional theory calculations. J. Chem. Phys. 2014, 140, 244307. [Google Scholar] [PubMed]
- Danten, Y.; Cabaço, I.; Coutinho, J.A.P.; Pinaud, N.; Besnard, M. DFT Study of the Reaction Mechanisms of Carbon Dioxide and its Isoelectronic Molecules CS2 and OCS Dissolved in Pyrrolidinium and Imidazolium Acetate Ionic Liquids. J. Phys. Chem. B 2016, 120, 5243–5254. [Google Scholar] [CrossRef] [PubMed]
- Cabaço, M.I.; Besnard, M.; Vaca-Chávez, F.; Pinaud, N.; Sebastião, P.J.; Coutinho, J.A.P.; Mascetti, J.; Danten, Y. On the chemical reactions of carbon dioxide isoelectronic molecules CS2 and OCS with 1-butyl-3-methylimidazolium acetate. Chem. Commun. 2013, 49, 11083–11085. [Google Scholar] [CrossRef] [PubMed]
- Gattow, G.; Behrendt, W. Carbon Sulfides and Their Inorganic and Complex Chemistry; Thieme: Stuttgart, Germany, 1977; p. 261. [Google Scholar]
- James, C.M. Synthesis and Characterisation of Inorganic Trithiocarbonates, Perthiocarbonates and Related 1,1-Dithiolate Compounds. Ph.D. Thesis, University of Cologne, Cologne, Germany, 2021; p. 295. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Dean, P.M.; Pringle, J.M.; MacFarlane, D.R. 1-Methyl-1-propylpyrrolidinium chloride. Acta Cryst. 2008, E64, o637. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E. Crystal structures of (Et4N)2C2S6, (Et4N)2C2S5, (Et4N)2CS4, (Et4N)HCS3, (Et4N)2CS3·2H2Oand(Et4N)2CS4-y·yH2O (y=0.69). Z. Anorg. Allg. Chem. 2021, 647, 777–782. [Google Scholar] [CrossRef]
- Silber, P.; Robineau, M.; Zins, D.; Brianso-Perucaud, M.C. Structure of a new perthiocarbonate anion, C2S62−, in the solid [(CH3)4N]2C2S6.1/2 CS2. Comptes Rendus Hebd. Seances Acad. Sci. Ser. C 1975, 280, 1517–1519. [Google Scholar]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; El-Katri, F.; Rehder, D.; Stammler, A.; Bögge, H.; Hellweg, F. Einfache Trithio- und Perthiocarbonatokomplexe mit interessanter Koordinationschemie: [E(CS3)2]2− (E = Sn, Zn, Cd), [E(CS3)3]3− (E = As, Sb, Bi, Co), {Cu(CS3)−}∞ und [Zn(CS4)2]2−. Z. Anorg. Allg. Chem. 1995, 621, 1160–1170. [Google Scholar] [CrossRef]
- Robineau, M.; Zins, D.; Brianso-Perucaud, M.-C. Crystal structure of a new type of perthiocarbonate, [(CH3)4N]2C2S6, 1/2 CS2. Rev. Chim. Miner. 1975, 12, 440–447. [Google Scholar]
- Samouei, H.; Reibenspies, J.H.; Darensbourg, D.J. Studies of the interactions of the tungsten pentacarbonyl fluoride anion with carbon dioxide. Polyhedron 2022, 221, 115852. [Google Scholar] [CrossRef]
- Kelly, R.P.; Falcone, M.; Alvarez Lamsfus, C.; Scopelliti, R.; Maron, L.; Meyer, K.; Mazzanti, M. Metathesis of a UV imido complex: A route to a terminal UV sulfide. Chem. Sci. 2017, 8, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Supplementary X-ray Crystallographic Data; Marchivie, M. (Eds.) Cambridge Crystallographic Data Centre, University Chemical Lab: Cambridge, UK, 2024; Available online: https://www.ccdc.cam.ac.uk/ (accessed on 20 June 2024).
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinaud, N.; Danten, Y.; Marchivie, M.; Besnard, M.; Cabaço, I.; Guillon, J. Crystal Structure of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate Complex. Molbank 2024, 2024, M1849. https://doi.org/10.3390/M1849
Pinaud N, Danten Y, Marchivie M, Besnard M, Cabaço I, Guillon J. Crystal Structure of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate Complex. Molbank. 2024; 2024(3):M1849. https://doi.org/10.3390/M1849
Chicago/Turabian StylePinaud, Noël, Yann Danten, Mathieu Marchivie, Marcel Besnard, Isabel Cabaço, and Jean Guillon. 2024. "Crystal Structure of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate Complex" Molbank 2024, no. 3: M1849. https://doi.org/10.3390/M1849
APA StylePinaud, N., Danten, Y., Marchivie, M., Besnard, M., Cabaço, I., & Guillon, J. (2024). Crystal Structure of Bis(1-butyl-1-methypyrrolidinium) Perthiodicarbonate Complex. Molbank, 2024(3), M1849. https://doi.org/10.3390/M1849






