Abstract
Tea is a daily drink for most people, and one of its major ingredients, epigallocatechin-3-gallate (EGCG), has been widely recognized as a potent antioxidant with diverse biological activities. However, its low stability and bioavailability hinder its further clinical applications. In this study, we designed and synthesized a novel EGCG-valine derivative 4 by replacing the gallic acid with a valine moiety in four steps. The structural elucidation of derivative 4 was performed using NMR, IR, mass, and UV spectroscopies. Additionally, the physicochemical properties of 4 were predicted by SwissADME, showing improved drug-like parameters and intestinal absorption compared to the parent compound EGCG.
1. Introduction
Epigallocatechin-3-gallate (EGCG) is a naturally occurring hydrophilic polyphenol, which is an active, abundant component found in tea. EGCG is a kind of catechin belonging to flavonoids and is predominant in green tea. Unlike green tea, black tea involves a fermentation process in its preparation, during which these phenolic compounds are oxidized to form new compounds, such as theobromine and theaflavin, thereby diminishing the EGCG content in black tea. EGCG exhibits a broad spectrum of biological activities, including hypoglycemic [1], antibacterial [2], antiviral [3], antioxidant [4], anti-atherosclerotic [5], anti-tumor [6] and anti-inflammatory [7] effects. Therefore, tea and its extract have been widely commercialized, commonly in the pharmaceutical, healthcare, and food industries [8,9,10]. However, its limitations, such as low bioavailability and low chemical and thermal stability, hinder its further clinical application [11,12].
From a structural perspective, EGCG is a polyphenolic compound consisting of a typical flavonoid moiety (two phenyl rings, A and B, and one dihydropyran ring, C) with a gallic acid group connected by an ester linkage at the C3 position (Figure 1). In addition, multiple hydroxyl groups at various positions on EGCG not only increase its water solubility but also make it versatile for chemical transformation. However, these hydroxyl groups also pose difficulties in selectively modifying EGCG’s structure. Typically, a protection-deprotection strategy is employed to avoid unnecessary side reactions.
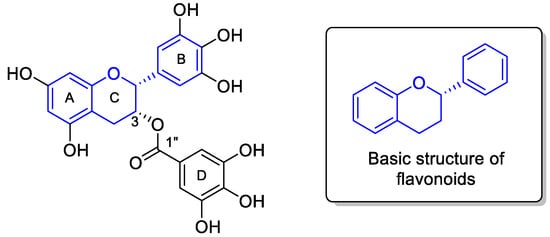
Figure 1.
Structure of EGCG.
Apart from synthetic challenges, the structural modification of EGCG is still considered an effective way to improve its stability. One study reported that the incorporation of d-glucose into EGCG yields glucosylated EGCG derivatives in low yields (<30%), demonstrating higher stability and water solubility but reduced antioxidant properties [13]. Moreover, lipophilic EGCG derivatives were synthesized with improved lipid solubility and antioxidant properties [14]. A major C3 modification of EGCG has also been reported via a five-step synthesis with a protection–deprotection strategy to afford promising derivatives with enhanced antioxidant effects [15].
Given the importance of EGCG in our daily products and its potential therapeutic use, we herein report the synthesis and characterization of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-yl l-valinate. This EGCG–valine hybrid compound may offer a potential direction for the modification of EGCG’s properties.
2. Results and Discussion
To improve the physicochemical properties of EGCG, we attempted to modify the gallic acid group to a valine (Val) moiety, where valine is a biocompatible scaffold with the ability to enhance bioavailability [16]. By modifying a similar synthetic strategy reported in the literature [15], we designed the synthesis of EGCG derivative 4 with the use of the O-methyl group due to its reaction tolerance as well as its enhancement in metabolic stability [17,18]. As depicted in Scheme 1, derivative 4 was prepared in four steps involving O-methylation, hydrolysis, esterification, and N-deprotection.
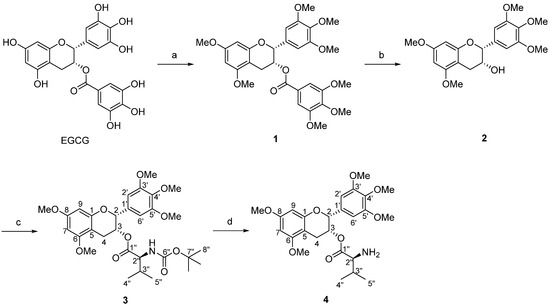
Scheme 1.
Synthetic routes of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-yl l-valinate from EGCG. (a) K2CO3, dimethyl sulfate, acetone, reflux; (b) K2CO3, methanol, reflux, 64% yield over 2 steps; (c) DCC, DMAP, Boc-Val-OH, CH2Cl2, r.t., 98% yield; and (d) TFA, CH2Cl2, r.t., 84% yield.
Briefly, all hydroxyl groups of EGCG were first protected upon treatment with dimethyl sulfate, resulting in the formation of intermediate 1. Its formation is recognized by the intense peaks of methoxy groups ranging from 3.71 to 3.86 ppm in the 1H NMR of a crude product (Supplementary Materials, Figure S1). Subsequently, under alkaline conditions, hydrolysis of intermediate 1 yielded intermediate 2, which illustrated the loss of the gallic acid group by the absence of an aromatic ring signal at 7.17 ppm and the corresponding three methoxy signals in 1H NMR spectrum (Supplementary Materials, Figure S3). Following that, the typical Steglich esterification of 2 and the subsequent N-Boc deprotection afforded the EGCG–Val hybrid compound a high yield. Finally, the desired compound 4 was subjected to characterization using NMR, IR, MS, and UV spectroscopies.
The structure of 4 was determined by 1H and 13C NMR spectra (Supplementary Materials, Figures S10 and S11). The 1H-NMR spectrum showed two doublets at 0.69 and 0.75 ppm corresponding to –CH3 protons on the isopropyl group and a doublet of the quintet at 1.80 ppm corresponding to the -CH- proton on the isopropyl group of valine residue (Supplementary Materials, Figure S10). These characteristic peaks indicate the incorporation of valine moiety into derivative 4.
For the 13C NMR signals, the appearance of the characteristic peak of quaternary carbon of the ester group at 174.7 ppm and the aliphatic carbon peaks at 17.0, 18.8, and 32.0 ppm proposed relevant characteristics to recognize the valine moiety in EGCG derivative (Supplementary Materials, Figure S11).
Heteronuclear single quantum coherence spectroscopy (HSQC), heteronuclear multiple bond correlation (HMBC), and 1H-1H correlated spectroscopy (COSY) were also used to assign 1H and 13C signals of compound 4, as shown in Table S1 (see Supplementary Materials for 2D spectra, Figures S12–S14).
The IR spectrum of compound 4 showed the following characteristic peaks: N–H stretching (weak) at 3387 cm−1, C–H stretching at 2954 and 2924 cm−1, C=O stretching of ester group at 1728 cm−1 and C=C stretching of aromatic rings at 1597 cm−1 (Supplementary Materials, Figure S15). Other vibrational peaks at 1458 cm−1 were attributed to the O–C bending in the methoxy groups. Moreover, strong absorption bands found at 1149 and 1100 cm−1 could be assigned to the characteristic C–O stretching vibrations of methoxy groups.
The UV spectrum of derivative 4 was also recorded for further characterization, showing an absorption peak at 209 nm and another lower absorption peak at 229 nm (Supplementary Materials, Figure S16).
Moreover, HRMS analysis was performed for 4, showing a good agreement with the theoretical mass (mass error <1 ppm). Therefore, these findings further support the proposed structure determined by NMR spectra (Supplementary Materials, Figure S18).
In addition, the physicochemical properties and oral bioavailability of derivatives 1 and 4 were predicted using the SwissADME website [19]. From the calculated physicochemical properties (Supplementary Materials, Table S2), compound 4 showed a greater improvement in its predicted physicochemical properties compared to parent compound EGCG and analog 1. Notably, O-methylation of the hydroxy groups increased iLogP significantly, which is a parameter representing the lipophilicity of the compound. Replacing gallic acid with valine moiety in 4 showed a decrease in iLogP, probably due to the incorporation of amine. In addition, these two derivatives exhibited no significant difference in TPSA. Overall, derivative 4 did not violate any of Lipinski’s rules, unlike its parent compound EGCG and analog 1 [20]. This indicates that the structural modification of EGCG into 4 improved the physiochemical properties greatly with regard to the drug-like characters.
Meanwhile, the BOILED-Egg model was also employed to evaluate the properties of 4, with the results showing a high probability of its absorption in the human intestine [21] (Supplementary Materials, Figure S20). Compound 4 was predicted to be orally bioavailable and absorbed in the human intestine by SwissADME [19].
3. Materials and Methods
3.1. General
All chemicals were purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Silica gel (particle size 230–400 mesh) was used for purification in column chromatography. Thin-layer chromatography was carried out using E. Merck precoated silica gel 60 F254 plates and visualized under a UV lamp.
1H-NMR, 13C-NMR, and 2D NMR spectra were acquired in CDCl3 at 25 °C on a Bruker Ascend®-600 NMR spectrometer (600 MHz for 1H and 150 MHz for 13C). All chemical shifts were reported in the standard δ notation of parts per million using TMS as an internal reference (δH 0 ppm; δC 0 ppm).
High-resolution mass spectroscopy (HRMS) analysis was conducted by an Aglient 1290 infinity system equipped with an Agilent 6230 electrospray ionization (ESI) time-of-flight (TOF) mass spectrometer using the UPLC column (Waters ACQUITY UPLC BEH C18, 100 mm × 2.1 mm, 1.7 μm). The measurements were conducted in a positive ion mode (interface capillary voltage 4500 V); the mass ratio was from m/z 50 to 3000 Da. The chromatographic conditions were as follows: mobile phase (acetonitrile/water with 0.1% formic acid), gradient elution, 20% acetonitrile/water for 2 min, 40% acetonitrile/water for 2 min, 60% acetonitrile/water for 2 min, 80% acetonitrile/water for 2 min, 100% acetonitrile/water for 3 min; flow rate 0.3 mL/min; and the injection volume was 1 μL. The purity of the compound was assessed by UPLC-UV analysis using an Aglient 1290 infinity system equipped with a 1290 DAD detector under the same chromatographic conditions.
UV analysis was performed by a Shimadzu UV—2600 with a 1 cm quartz cell and a slit width of 2.0 nm. The analysis was carried out using wavelength in the range of 200–400 nm.
3.2. Synthesis of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-yl 3,4,5-trimethoxybenzoate (1)
To a solution of EGCG (1.0211 g, 2.23 mmol) in acetone (11 mL), dimethyl sulfate (2.0 mL, 21.1 mmol) and K2CO3 (3.62 g, 26.2 mmol) were added. The reaction mixture was refluxed for 3 h. The reaction was diluted with acetone, filtered through celite, and concentrated in vacuo. The crude product of 1 was used directly in the next step without further purification. δH (600 MHz, CDCl3) 7.17 (s, 2H), 6.71 (s, 1H), 6.25 (d, J = 2.2 Hz, 1H), 6.13 (d, J = 2.2 Hz, 1H), 5.67 (t, J = 2.9 Hz, 1H), 5.09 (s, 1H), 3.86 (s, 2H), 3.81 (s, 3H), 3.80 (d, J = 2.1 Hz, 2H), 3.80 (s, 1H), 3.79 (s, 1H), 3.72 (s, 3H), 3.05 (d, J = 3.3 Hz, 1H) ppm; δC (150 MHz, CDCl3) 165.24, 159.85, 158.99, 155.59, 153.25, 152.97, 142.61, 137.98, 133.50, 125.20, 107.28, 103.99, 100.25, 93.35, 91.99, 77.89, 68.78, 61.00, 60.89, 56.36, 56.10, 55.52, 55.50, 26.04 ppm. These special characteristics are consistent with those reported in the literature [22].
3.3. Synthesis of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-ol (2)
To a solution of crude product 1 in methanol (22 mL) K2CO3 (362 mg, 2.62 mmol) was added. The reaction mixture was refluxed for 1.5 h. The reaction was diluted with CH2Cl2, filtered through celite, and concentrated in vacuo. The crude product was purified by silica column chromatography (eluents: 1–2% MeOH/CH2Cl2) to afford title compound 2 as a white solid (537.2 mg, 64% over 2 steps). δH (600 MHz, CDCl3) 6.75 (s, 2H), 6.21 (d, J = 2.3 Hz, 1H), 6.13 (d, J = 2.3 Hz, 1H), 4.94 (s, 1H), 4.29 (s, 1H), 3.90 (s, 6H), 3.86 (s, 3H), 3.80 (s, 3H), 3.78 (s, 3H), 2.98–2.94 (m, 1H), 2.89 (dd, J = 17.2, 4.4 Hz, 1H), 1.82 (d, J = 5.4 Hz, 1H) ppm; δC (150 MHz, CDCl3) 159.71, 159.28, 155.05, 153.48, 137.70, 133.97, 103.30, 100.26, 93.37, 92.28, 78.67, 66.49, 60.84, 56.22, 55.49, 55.40, 28.08 ppm. These special characteristics are consistent with those reported in the literature [23].
3.4. Synthesis of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-yl (tert-butoxycarbonyl)-l-valinate (3)
To a solution of intermediate 2 (50.3 mg, 0.134 mmol) in CH2Cl2 (8.9 mL), DCC (81.9 mg, 0.397 mmol), DMAP (19.5 mg, 0.160 mmol) and Boc-Val-OH (88.2 mg, 0.406 mmol) were added. The reaction mixture was stirred at room temperature overnight. The reaction was filtered through a short pad of silica gel, washed with CH2Cl2, and concentrated in vacuo. The crude product was purified by silica column chromatography (eluents: 25% EtOAc /Hex) to afford title compound 3 as a colorless oil (75.6 mg, 98%). Rf (30% EtOAc/Hex) 0.56; δH (600 MHz, CDCl3) 6.69 (s, 2H, H-2′/6′), 6.21 (d, J = 2.2 Hz, 1H, H-9), 6.10 (d, J = 2.2 Hz, 1H, H-7), 5.46 (s, 1H, H-3), 5.03 (s, 1H, H-2), 4.88 (d, J = 8.8 Hz, 1H, N-H), 4.09 (dd, J = 8.7, 4.6 Hz, 1H, H-2″), 3.88 (s, 6H, 3′/5′-OMe), 3.84 (s, 3H, 4′-OMe), 3.79 (s, 3H, 8-OMe), 3.77 (s, 3H, 6-OMe), 2.95 (s, 2H, H-4), 1.91 (td, J = 12.9, 6.5 Hz, 1H, H-3″), 1.36 (s, 9H, H-8″), 0.72 (d, J = 6.8 Hz, 3H, H-4″ or -5″), 0.64 (d, J = 6.9 Hz, 3H, H-4″ or -5″) ppm; δC (150 MHz, CDCl3) 171.45 (1″), 159.75 (8), 158.81 (6), 155.36 (6″), 155.20 (1), 153.27 (2C, 3′/5′), 137.83 (4′), 133.13 (1′), 103.55 (2C, 2′/6′), 99.68 (5), 93.27 (9), 92.04 (7), 79.65 (7″), 77.38 (2), 68.96 (3), 60.83 (4′-OMe), 58.60 (2″), 56.21 (2C, 3′/5′-OMe), 55.44 (6- or 8-OMe), 55.38 (6- or 8-OMe), 31.43 (3″), 28.21 (3C, 8″), 25.68 (4), 18.37 (4″ or 5″), 17.24 (4″ or 5″) ppm; HRMS-ESI m/z 598.2626 [M + Na]+ (calcd. for C30H41NO10, m/z 598.2623).
3.5. Synthesis of (2R,3R)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chroman-3-yl l-valinate (4)
To a solution of intermediate 3 (52.7 mg, 0.0915 mmol) in CH2Cl2 (1 mL), TFA (57 µL) was added. The reaction mixture was then stirred at room temperature overnight. The reaction was quenched with K2CO3, then filtered off the solid and concentrated in vacuo. The crude product was purified by silica column chromatography (eluents: 50% EtOAc/Hex with 1% Et3N) to afford desired product 4 as a colorless oil (36.5 mg, 84%). Rf (50% EtOAc/Hex with 1% Et3N) 0.34; IR (ATR, νmax/cm−1): 3387, 2954, 2924, 1728, 1597, 1458, 1149, 1100; δH (600 MHz, CDCl3) 6.70 (s, 2H, H-2′/6′), 6.21 (d, J = 2.3 Hz, 1H, H-9), 6.11 (d, J = 2.3 Hz, 1H, H-7), 5.46 (t, J = 2.7 Hz, 1H, H-3), 5.04 (s, 1H, H-2), 3.88 (s, 6H, 3′/5′-OMe), 3.84 (s, 3H, 4′-OMe), 3.79 (s, 3H, 8-OMe), 3.78 (s, 3H, 6-OMe), 3.12 (t, J = 5.4 Hz, 1H, H-2″), 2.96 (d, J = 3.2 Hz, 2H, H-4), 1.80 (qd, J = 12.1, 6.8 Hz, 1H, H-3″), 0.75 (d, J = 6.9 Hz, 3H, H-4″ or -5″), 0.69 (d, J = 6.9 Hz, 3H, H-4″ or -5″) ppm; δC (150 MHz, CDCl3) 174.67 (1″), 159.70 (8), 158.85 (6), 155.18 (1), 153.19 (2C, 3′/5′), 137.76 (4′), 133.36 (1′), 103.56 (2C, 2′/6′), 99.81 (5), 93.32 (9), 92.01 (7), 77.37 (2), 68.40 (3), 60.85 (4′-OMe), 59.94 (2″), 56.21 (2C, 3′/5′-OMe), 55.48 (8-OMe), 55.38 (6-OMe), 32.03 (3″), 25.72 (4), 18.81 (4″ or 5″), 16.99 (4″ or 5″) ppm; HRMS-ESI m/z 476.2282 [M + H]+ (calcd. for C25H33NO8, m/z 476.2279); = −88.0 (0.000136, EtOH); UV (EtOH, λmax) 209 and 229 nm. The purity of 4 was assessed by UPLC-UV at 254 nm to be >95%.
4. Conclusions
A hybrid compound of EGCG and valine was synthesized in four steps. The gallic acid moiety was substituted by a valine moiety at the C3 position of EGCG via an ester linkage. This novel derivative 4 was fully characterized by NMR, IR, mass, and UV spectroscopies. An in silico analysis of its physicochemical properties was performed, demonstrating improved drug-like parameters and better intestinal absorption in comparison with the parent compound EGCG.
Supplementary Materials
Figures S1–S14: NMR spectra; Figure S15: IR spectrum of compound 4; Figure S16: UV spectrum of compound 4; Figures S17 and S18: mass spectra; Figure S19: UPLC-UV chromatogram of 4; Figure S20: BOILED-Egg graph resuming the predicted properties for EGCG and derivative 4; Table S1: Assignment of 1H and 13C NMR chemical shifts of 4; Table S2: Physicochemical properties of EGCG and derivatives 1 and 4 calculated by SwissADME.
Author Contributions
Synthesis, X.Y.; analysis, Z.R.; writing—original draft preparation, X.Y. and Z.R.; writing—review and editing, P.C. and J.P.L.N.; supervision, P.C. and J.P.L.N.; project administration, P.C. and J.P.L.N.; funding acquisition, P.C. and J.P.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Science and Technology Development Fund, Macau SAR (File no.: 006/2023/SKL, 002/2023/ALC and 0005-2023-RIA1 (PC)).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ma, R.; Sun, W.; Ji, Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. Int. J. Environ. Res. Public Health 2023, 20, 4676. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Li, Q.-S.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef]
- Messire, G.; Serreau, R.; Berteina-Raboin, S. Antioxidant Effects of Catechins (EGCG), Andrographolide, and Curcuminoids Compounds for Skin Protection, Cosmetics, and Dermatological Uses: An Update. Antioxidants 2023, 12, 1317. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.-Z.; Wu, Y.; Wang, R.-Q.; Chen, J.-W.; Chen, J.; Zhang, Y.; Chen, Y.-J.; Geng, M.; Xu, Z.-D.; et al. (–)-Epigallocatechin-3-Gallate Ameliorates Atherosclerosis and Modulates Hepatic Lipid Metabolic Gene Expression in Apolipoprotein E Knockout Mice: Involvement of TTC39B. Front. Pharmacol. 2018, 9, 195. [Google Scholar] [CrossRef]
- Luo, K.-W.; Zhu, X.-H.; Zhao, T.; Zhong, J.; Gao, H.-C.; Luo, X.-L.; Huang, W.-R. EGCG Enhanced the Anti-tumor Effect of Doxorubicine in Bladder Cancer via NF-κB/MDM2/p53 Pathway. Front. Cell Dev. Biol. 2020, 8, 606123. [Google Scholar] [CrossRef]
- Payne, A.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Molecular Mechanisms of the Anti-Inflammatory Effects of Epigallocatechin 3-Gallate (EGCG) in LPS-Activated BV-2 Microglia Cells. Brain Sci. 2023, 13, 632. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and Antimicrobial Activities of (-)-Epigallocatechin-3-gallate (EGCG) and its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Shivaji, K.; Mani, S.; Balasubramanian Mythili, G. Tea Polyphenols Chemistry for Pharmaceutical Applications. In Tea; Gonçalo, J., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Ji, F.; Liu, H.; Wang, C.; Guo, N.; Shen, Y.; Luo, S.; Jiang, S.; Zheng, Z. Remodeling the structure of soy protein fibrils to hydrogels for co-encapsulation of (−)-epigallocatechin gallate (EGCG) and curcumin: Role of EGCG. Food Hydrocoll. 2024, 147, 109439. [Google Scholar] [CrossRef]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Song, X.; Du, J.; Zhao, W.; Guo, Z. Epigallocatechin-3-gallate(EGCG): Mechanisms and the Combined Applications. Comb. Chem. High Throughput Screen. 2017, 20, 872–885. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Hu, J.-M.; Huang, Y.-W.; Wu, X.-Y.; Zi, C.-T.; Wang, X.-J.; Sheng, J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef]
- Wang, S.; Jin, R.; Wang, R.; Hu, Y.; Dong, X.; Xu, A.e. The design, synthesis and biological evaluation of pro-EGCG derivatives as novel anti-vitiligo agents. RSC Adv. 2016, 6, 106308–106315. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, H.; Li, X.; Quan, Z.-S. Application of Amino Acids in the Structural Modification of Natural Products: A Review. Front. Chem. 2021, 9, 650569. [Google Scholar] [CrossRef]
- Sun, S.; Fu, J. Methyl-containing pharmaceuticals: Methylation in drug design. Bioorg. Med. Chem. Lett. 2018, 28, 3283–3289. [Google Scholar] [CrossRef]
- Pinheiro, P.d.S.M.; Franco, L.S.; Fraga, C.A.M. The Magic Methyl and Its Tricks in Drug Discovery and Development. Pharmaceuticals 2023, 16, 1157. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V.A. Boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Kitade, M.; Ohno, Y.; Tanaka, H.; Takahashi, T. An Efficient Synthesis of (±)-Epigallocatechin Gallate by Reductive Intramolecular Etherification. Synlett 2006, 2006, 2827–2829. [Google Scholar] [CrossRef]
- Liu, Z.; Fukagawa, Y.; Yamano, M.; Tago, T.; Iwai, K.; Hirano, K.; Kumazoe, M.; Tachibana, H.; Toyohara, J.; Tanaka, H. A gold-complex initiated functionalization of biologically active polyphenols applied to a 18F-labeled chemical probe. Org. Biomol. Chem. 2023, 21, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).