(Hetero)Arene Ring-Fused [1,2,4]Triazines
Abstract
1. Introduction
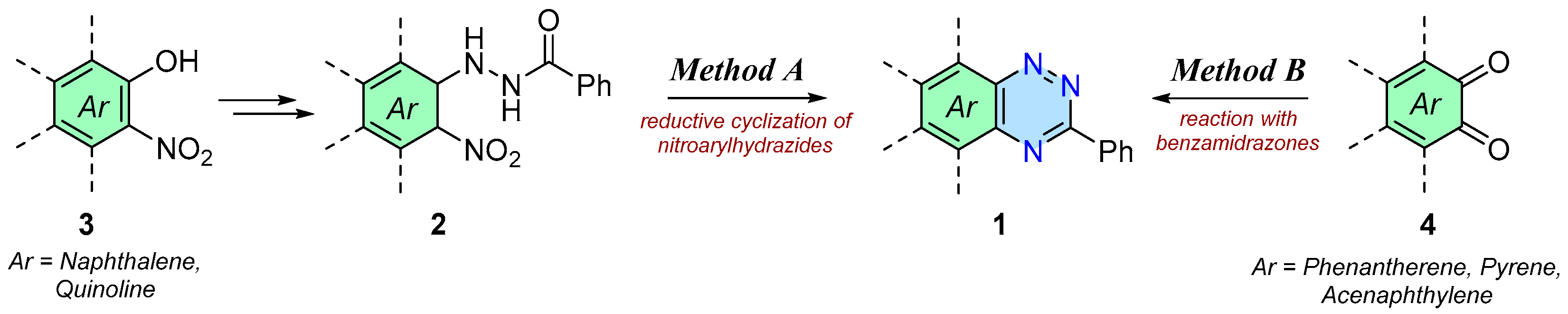
2. Results and Discussion
3. Materials and Methods
3.1. General Information
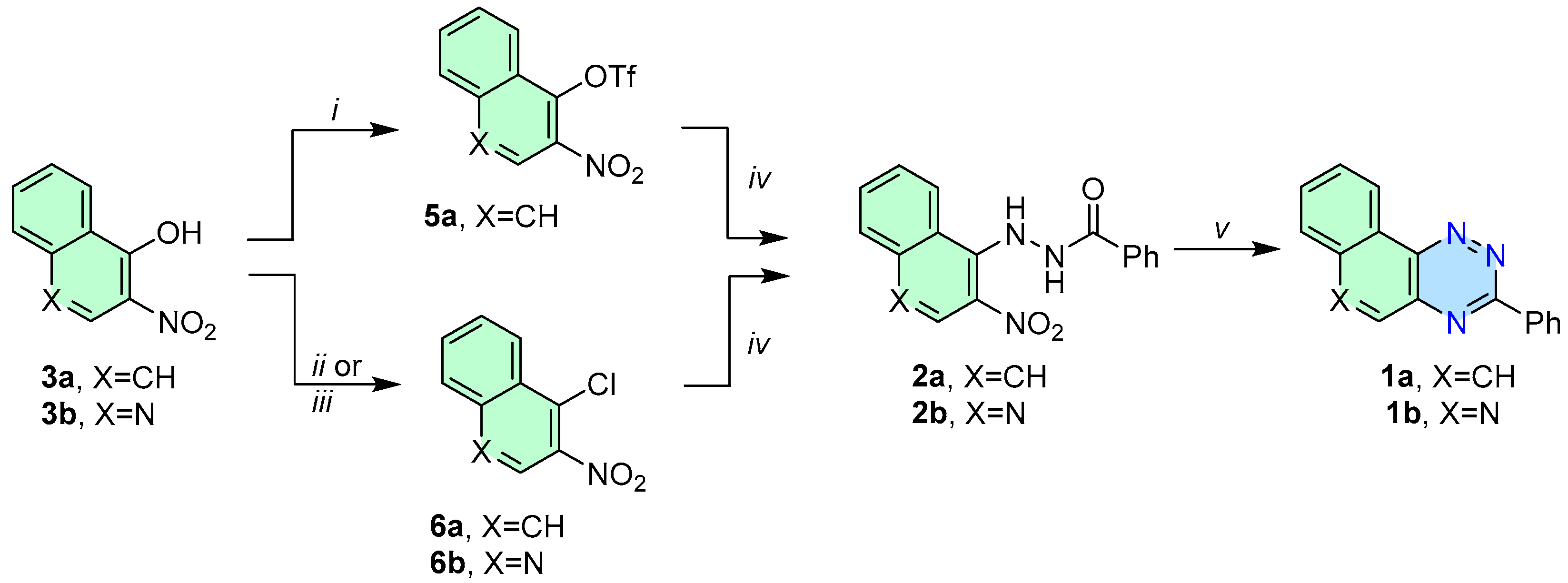
3.2. General Procedure for the Synthesis of Triazines 1a–1b—Method A
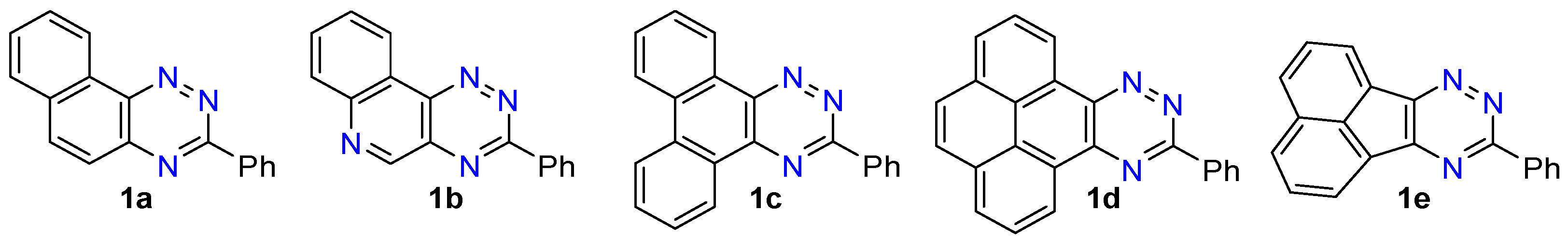
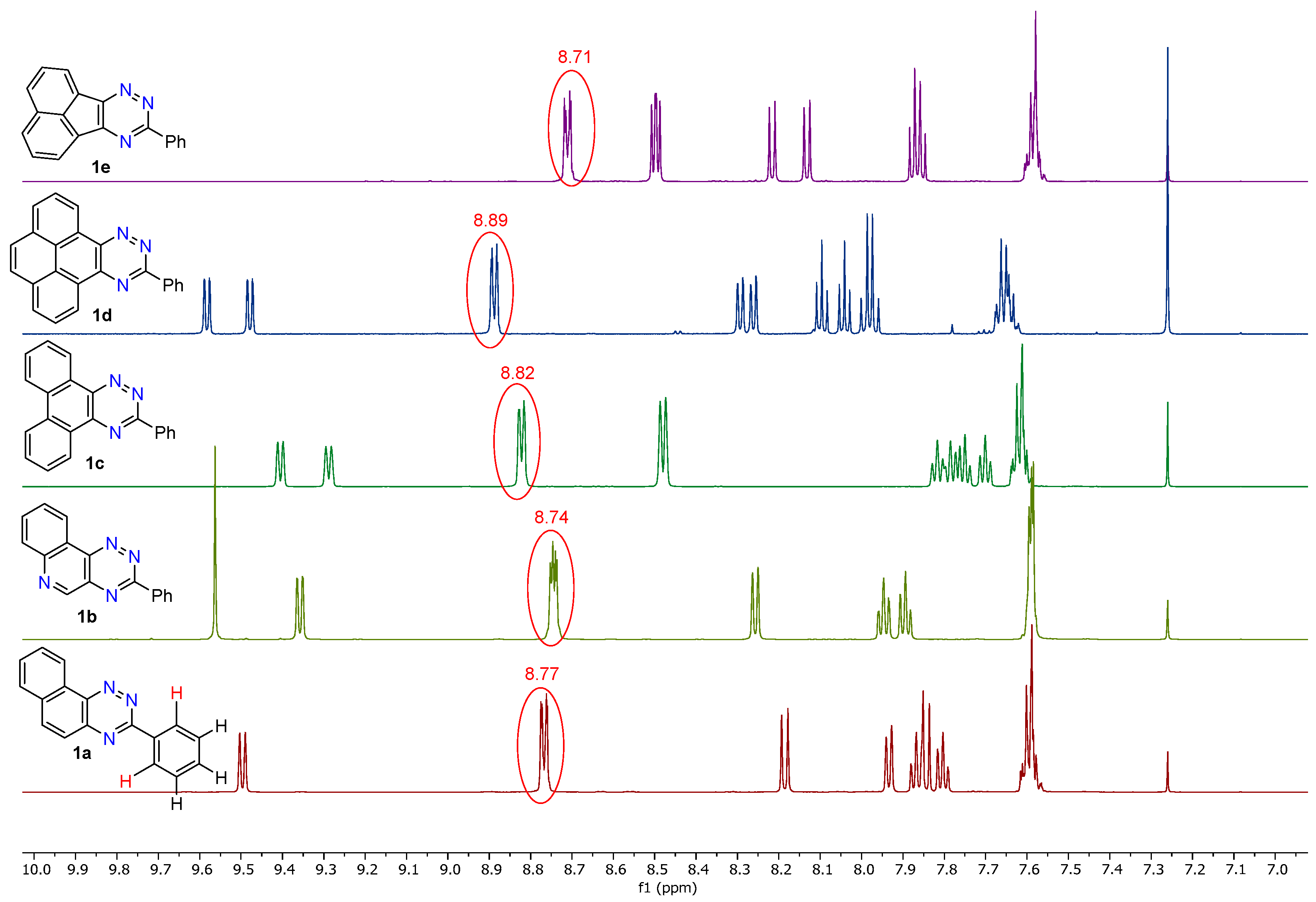
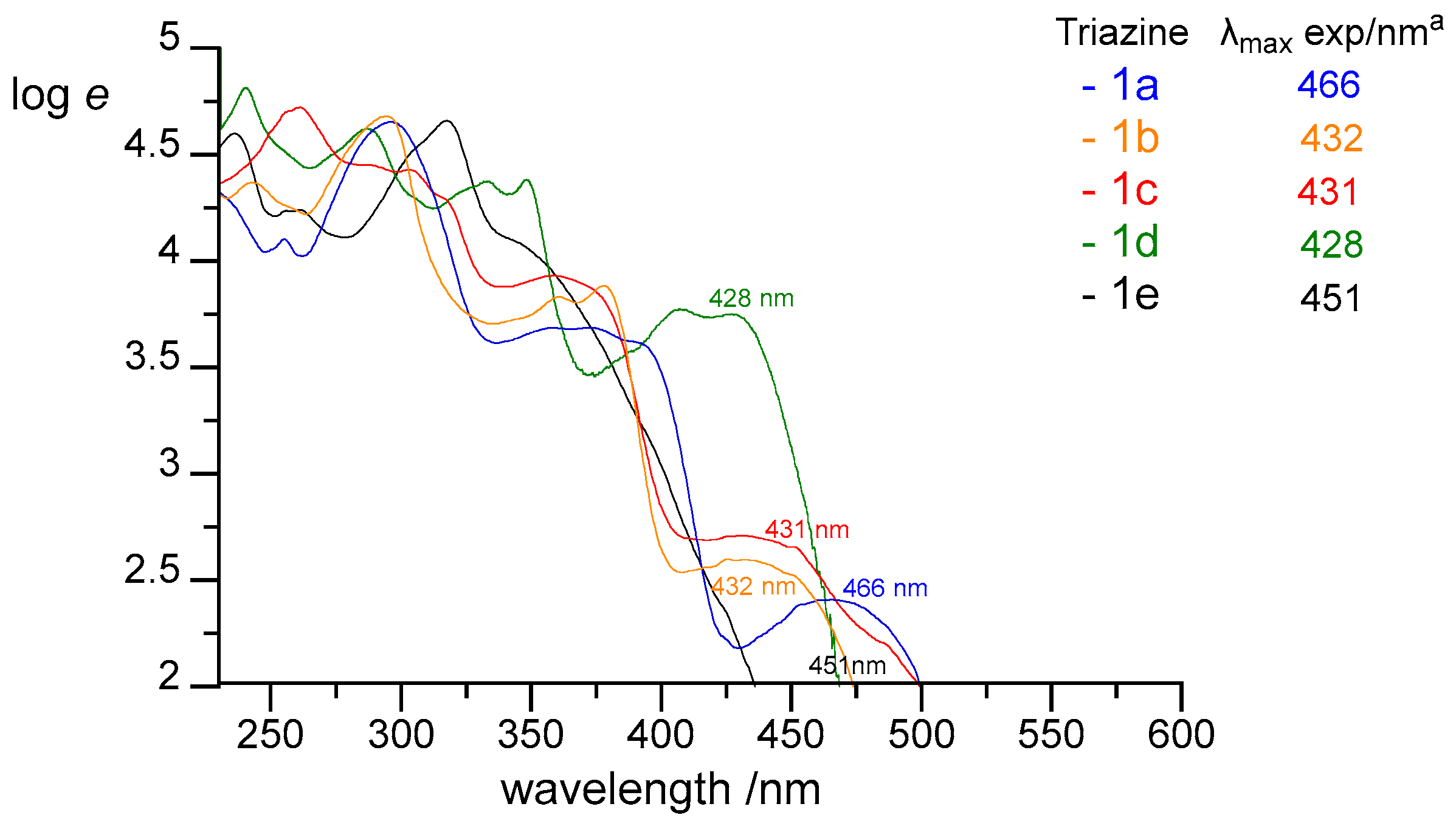
- 3-Phenylnaphtho[2,1-e][1,2,4]triazine (1a). 360–378 mg (80–84% yield) as a yellow solid: mp 135–140 °C; 1H NMR (600 MHz, CDCl3) δ 9.50 (d, J = 8.1 Hz, 1H), 8.77 (dt, J1 = 8.4 Hz, J2 = 2.3 Hz, 2H), 8.19 (d, J = 9.1 Hz, 1H), 7.93 (d, J = 7.8 Hz, 1H), 7.89–7.83 (m, 2H), 7.83–7.78 (m, 1H), 7.65–7.51 (m, 3H); 13C{1H} NMR (151 MHz, CDCl3) δ 161.4, 144.9, 143.1, 138.3, 135.7, 132.8, 131.5, 130.2, 129.4, 129.4, 129.0, 128.6, 125.8, 124.0; IR (KBr) ν 1599, 1518, 1434, 1384, 1276, 1219, 1160, 1048, 927, 849, 738, 689, 544 cm−1; UV (CH2Cl2) λmax (log ε) 295 (4.65), 358 (3.68), 373 (3.69), 388 (3.62 sh), 466 (2.41) nm; HRMS (ESI) [M + H]+ m/z calcd for C17H12N3: 258.1031; found: 258.1029. Anal. Calcd for C17H11N3: C, 79.36; H, 4.31; N, 16.33. Found: C, 79.15; H, 4.27; N, 16.44.
- 3-Phenyl-[1,2,4]triazino[5,6-c]quinoline (1b). 227–248 mg (50–55% yield) as orange needles: mp 204–205 °C; 1H NMR (600 MHz, CDCl3) δ 9.56 (s, 1H), 9.36 (dd, J1 = 8.0 Hz, J2 = 1.3 Hz, 1H), 8.74 (dd, J1 = 6.7 Hz, J2 =3.0 Hz, 2H), 8.26 (d, J = 8.1 Hz, 1H), 7.97–7.93 (m, 1H), 7.92–7.87 (m, 1H), 7.63–7.53 (m, 3H); 13C{1H} NMR (151 MHz, CDCl3) δ 162.7, 154.8, 145.8, 144.6, 134.8, 134.6, 132.2, 132.0, 130.3, 129.9, 129.2, 128.7, 123.2, 121.7; IR (KBr) ν 1607, 1509, 1419, 1376, 1271, 1120, 1004, 931, 853, 766, 688, 563 cm−1; UV (CH2Cl2) λmax (log ε) 296 (4.72), 360 (3.83), 378 (3.88), 432 (2.60) nm; HRMS (ESI) [M + H]+ m/z calcd for C16H11N4: 259.0984; found: 259.0988. Anal. Calcd for C16H10N4: C, 74.40; H, 3.90; N, 21.69. Found: C, 74.53; H, 3.92; N, 21.80.
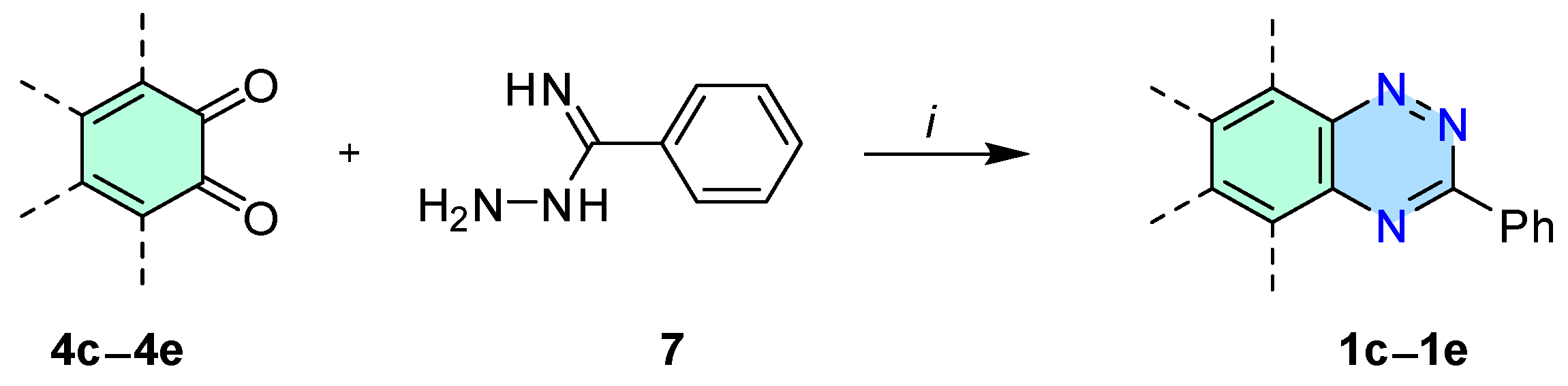
3.3. General Procedure for Synthesis of Triazines 1c–1e—Method B
- 3-Phenylphenanthro[9,10-e][1,2,4]triazine (1c). 266 mg (86% yield) as a yellow powder: mp 175–180 °C; 1H NMR (600 MHz, CDCl3) δ 9.41 (d, J = 7.8 Hz, 1H), 9.29 (d, J = 7.9 Hz, 1H), 8.87–8.79 (m, 2H), 8.48 (d, J = 8.0 Hz, 2H), 7.85–7.73 (m, 3H), 7.70 (t, J = 7.5 Hz, 1H), 7.66–7.57 (m, 3H); 13C{1H} NMR (151 MHz, CDCl3) δ 161.3, 144.8, 143.0, 135.8, 133.9, 132.4, 131.5, 131.0, 130.7, 129.0, 128.7, 128.5, 128.2, 128.1, 127.7, 126.6, 124.9, 123.1, 123.1; IR (KBr) ν 1607, 1508, 1448, 1408, 1369, 1279, 1167, 1080, 960, 870, 758, 690, 541, 431 cm−1; UV (CH2Cl2) λmax (log ε) 261 (4.72), 289 (4.45), 303 (4.43), 316 (4.30 sh), 431 (2.70) nm; HRMS (ESI) [M + H]+ m/z calcd for C21H14N3: 308.1188; found: 308.1191. Anal. Calcd for C21H13N3: C, 82.06; H, 4.26; N, 13.67. Found: C, 82.16; H, 4.26; N, 13.49.
- 3-Phenylpyreno[9,10-e][1,2,4]triazine (1d). 242 mg (73% yield) as a yellow solid: mp 246–247 °C; 1H NMR (600 MHz, CDCl3) δ 9.58 (dd, J1 = 7.6 Hz, J2 = 0.9 Hz, 1H), 9.48 (dd, J1 = 7.6 Hz, J2 = 0.9 Hz, 1H), 8.89 (dt, J1 = 8.4 Hz, J2 = 2.1 Hz, 2H), 8.28 (dd, J1 = 19.3 Hz, J2 = 7.6 Hz, 2H), 8.10 (t, J = 7.7 Hz, 1H), 8.04 (t, J = 7.6 Hz, 1H), 7.98 (q, J = 8.9 Hz, 2H), 7.70–7.58 (m, 3H); 13C{1H} NMR (151 MHz, CDCl3) δ 161.6, 145.7, 144.0, 135.8, 131.6, 131.4, 131.3, 131.1, 129.4, 129.1, 128.6, 127.7, 127.5, 127.3, 127.3, 127.2, 126.9, 126.8, 125.2, 124.6, 122.6; IR (KBr) ν 3049, 1625, 1492, 1444, 1364, 1294, 1228, 1175, 1053, 928, 831, 769, 697 cm−1; UV (CH2Cl2) λmax (log ε) 240 (4.81), 287 (4.62), 333 (4.37), 348 (4.38), 407 (3.77), 428 (3.75) nm; HRMS (ESI) [M + H]+ m/z calcd for C23H14N3: 332.1188; found: 332.1186. Anal. Calcd for C23H13N3: C, 82.86; H, 4.54; N, 12.60. Found: C, 82.82; H, 4.27; N, 12.85.
- 9-Phenylacenaphtho[1,2-e][1,2,4]triazine (1e). 121 mg (43% yield) as a yellow solid; 1H NMR (600 MHz, CDCl3) δ 8.74–8.68 (m, 2H), 8.53–8.47 (m, 2H), 8.22 (d, J = 8.2 Hz, 1H), 8.13 (d, J = 8.2 Hz, 1H), 7.86 (dt, J = 8.2, 7.2 Hz, 2H), 7.62–7.54 (m, 3H); 13C{1H} NMR (151 MHz, CDCl3) δ 161.5, 157.7, 155.1, 136.0, 134.3, 132.3, 131.4, 130.2, 130.1, 130.0, 129.5, 129.1, 128.9, 128.8, 128.5, 125.2, 123.6; IR (KBr) ν 1617, 1565, 1528, 1479, 1382, 1353, 1207, 1159, 1110, 1028, 831, 775, 705; UV (CH2Cl2) λmax (log ε) 318 (4.66), 347 (4.08 sh), 451 (1.47) nm; HRMS (ESI) [M + H]+ m/z calcd for C19H12N3: 282.1031; found: 282.1033. Anal. Calcd for C19H11N3: C, 81.12; H, 3.94; N, 14.94. Found: C, 81.07; H, 3.89; N, 14.93.
3.4. General Procedure for the Synthesis of Hydrazides 2
- N′-(2-Nitronaphthalen-1-yl)benzohydrazide (2a). A mixture of compound 5 or 6 (2.0 mmol) and benzhydrazide (1 eq, 2.0 mmol) in DMSO (8 mL) was stirred at 65 °C under an argon atmosphere overnight. The mixture was cooled and poured into water (50 mL). The resulting yellow solid was filtered, washed with water, and dried. The crude product was purified by silica column chromatography (50% DCM/pet. ether) and recrystallized (EtOH) to give 553 mg (90% yield) of 2a from 5a, and 424 mg (69% yield) of 2a from 6a. Yellow solid: mp 195–200 °C; 1H NMR (600 MHz, DMSO–d6) δ 11.03 (s, 1H), 9.32 (s, 1H), 8.74 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 9.1 Hz, 1H), 7.76 (d, J = 7.4 Hz, 2H), 7.69 (t, J = 7.4 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.55 (t, J = 9.1 Hz, 2H), 7.46 (t, J = 7.6 Hz, 2H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 166.7, 142.8, 135.8, 134.7, 132.0, 131.9, 129.5, 128.5, 127.3, 126.5, 125.8, 125.0, 121.4, 120.6; IR (KBr) ν 3260, 1648, 1578, 1515, 1457, 1393, 1303, 1211, 1140, 1092, 1025, 903, 812, 761, 689 cm−1; HRMS (ESI) [M − H]− m/z calcd for C17H12N3O3: 306.0879; found: 306.0884. Anal. Calcd for C17H13N3O3: C, 66.44; H, 4.26; N, 13.67. Found: C, 66.30; H, 4.42; N, 13.52.
- N’-(3-Nitroquinolin-4-yl)benzohydrazide (2b). 573 mg (93% yield) of 2b from 6b. Yellow solid: mp 228–229 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.29 (s, 1H), 8.86 (s, 1H), 8.06–7.97 (m, 2H), 7.84 (d, J = 7.6 Hz, 2H), 7.78 (t, J = 7.6 Hz, 1H), 7.62 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.6 Hz, 2H); 13C{1H} NMR (151 MHz, DMSO-d6) δ 168.0, 166.2, 142.4, 138.4, 133.5, 133.1, 131.0, 130.5, 129.0, 128.9, 128.2, 127.9, 127.7, 126.1, 119.7; IR (KBr) ν 3100, 3007, 2944, 2796, 1987, 1687, 1595, 1526, 1348, 1265, 1077, 1023, 880, 840, 754, 690, 608, 532, 445 cm−1; HRMS (ESI) [M + H]+ m/z calcd for C16H13N4O3: 309.0988; found: 309.0991. Anal. Calcd for (C16H12N4O3)2H2O: C, 60.57; H, 4.13; N, 17.66. Found: C, 56.69; H, 3.88; N, 16.60.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yurttaş, L.; Demirayak, Ş.; Ilgin, S.; Atli, Ö. In Vitro Antitumor Activity Evaluation of Some 1,2,4-Triazine Derivatives Bearing Piperazine Amide Moiety against Breast Cancer Cells. Bioorganic Med. Chem. 2014, 22, 6313–6323. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. An Overview on the Recent Developments of 1,2,4-Triazine Derivatives as Anticancer Compounds. Eur. J. Med. Chem. 2017, 142, 328–375. [Google Scholar] [PubMed]
- Sztanke, K.; Pasternak, K.; Rajtar, B.; Sztanke, M.; Majek, M.; Polz-Dacewicz, M. Identification of Antibacterial and Antiviral Activities of Novel Fused 1,2,4-Triazine Esters. Bioorganic Med. Chem. 2007, 15, 5480–5486. [Google Scholar] [CrossRef]
- Arshad, M.; Bhat, A.R.; Hoi, K.K.; Choi, I.; Athar, F. Synthesis, Characterization and Antibacterial Screening of Some Novel 1,2,4-Triazine Derivatives. Chin. Chem. Lett. 2017, 28, 1559–1565. [Google Scholar]
- Khoshneviszadeh, M.; Ghahremani, M.H.; Foroumadi, A.; Miri, R.; Firuzi, O.; Madadkar-Sobhani, A.; Edraki, N.; Parsa, M.; Shafiee, A. Design, Synthesis and Biological Evaluation of Novel Anti-Cytokine 1,2,4-Triazine Derivatives. Bioorganic Med. Chem. 2013, 21, 6708–6717. [Google Scholar] [CrossRef] [PubMed]
- Rusinov, V.L.; Egorov, I.N.; Chupakhin, O.N.; Belanov, E.F.; Bormotov, N.I.; Serova, O.A. The Search for New Drugs: Synthesis and Antiviral Activity of 1,2,4-Triazine Derivatives. Pharm. Chem. J. 2012, 45, 655–659. [Google Scholar] [CrossRef]
- Branowska, D.; Olender, E.; Wysocki, W.; Karczmarzyk, Z.; Bancerz, I.; Ledwon, P.; Lapkowski, M.; Mirosław, B.; Urbańczyk-Lipkowska, Z.; Kalicki, P. Synthesis and Electrochemical Characterization of Oligothiophenes with 1,2,4-Triazine and 5,5′-Bi-1,2,4-Triazine as Strong Electron Acceptor Units. Electrochim. Acta 2016, 214, 19–30. [Google Scholar] [CrossRef]
- Maggiore, A.; Tan, X.; Brosseau, A.; Danos, A.; Miomandre, F.; Monkman, A.P.; Audebert, P.; Clavier, G. Novel D-A Chromophores with Condensed 1,2,4-Triazine System Simultaneously Display Thermally Activated Delayed Fluorescence and Crystallization-Induced Phosphorescence. Phys. Chem. Chem. Phys. 2022, 24, 17770–17781. [Google Scholar] [PubMed]
- Sakr, M.A.; Kana, M.T.A. 1,2,4-Triazine-Based Materials: Spectroscopic Investigation, DFT, NBO, and TD-DFT Calculations as Well As Dye-Sensitized Solar Cells Applications. J. Fluoresc. 2022, 32, 2053–2063. [Google Scholar] [CrossRef]
- Nagy, J.; Nyitrai, J.; Kolonits, P.; Lempert, K.; Gergely, A.; Párkányi, L.; Kálmán, A. Photochemistry of N-Heterocycles. Part 1. Synthesis and Photochemistry of Some 2,5-Dihydro-1,2,4-Triazines. X-ray Molecular Structure of 1-(4-Methyl-3,5,6-Triphenyl-1,4,5,6-Tetrahydro-1,2,4-Triazin-6-Yl)Ethanol. J. Chem. Soc. Perkin Trans. 1988, 12, 3267–3274. [Google Scholar] [CrossRef]
- Wright, G.C.; Gray, J.E.; Yu, C.N. Synthesis and Antifungal Properties of 3-Substituted Os-Triazino[5,6-c]Quinolines. J. Med. Chem. 1974, 17, 244–246. [Google Scholar] [CrossRef]
- Bennett, G.B.; Mason, R.B.; Alden, L.J.; Roach, J.B. Synthesis and Antiinflammatory Activity of Trisubstituted Pyrimidines and Triazines. J. Med. Chem. 1978, 486, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hajpal, I.; Berenyi, E. Synthesis of As-Triazono[5,6-b]Quinoline, a New Heterocyclic Ring System. J. Heterocycl. Chem. 1982, 19, 313–315. [Google Scholar] [CrossRef]
- Berényi, E.; Benkó, P.; Pallos, L. Synthesis of As-Triazno[5,6-c]Quinolines and Its Derivatives. Acta Chim. Acad. Sci. Hung. 1976, 90, 399–404. [Google Scholar]
- Berezin, A.A.; Zissimou, G.; Constantinides, C.P.; Beldjoudi, Y.; Rawson, J.M.; Koutentis, P.A. Route to Benzo- and Pyrido-Fused 1,2,4-Triazinyl Radicals via N′-(Het)Aryl-N′-[2-Nitro(Het)Aryl]Hydrazides. J. Org. Chem. 2014, 79, 314–327. [Google Scholar] [CrossRef]
- Azizian, J.; Krimi, A.R. Synthesis of Trisubstituted 1,2,4-Triazines in Presence of NaHSO4/SiO2. Asian J. Chem. 2011, 23, 980–982. [Google Scholar]
- Linsong, C.; Xiangdong, Z.; Yexin, Z.; Hua, C. Polycyclic Aromatic Hydrocarbon Aza-Naphthalene Derivative, Synthesis Method and Electronic Device Thereof. CN111548350A, 16 April 2020. [Google Scholar]
- Crespin, L.; Biancalana, L.; Morack, T.; Blakemore, D.C.; Ley, S.V. One-Pot Acid-Catalyzed Ring-Opening/Cyclization/Oxidation of Aziridines with N-Tosylhydrazones: Access to 1,2,4-Triazines. Org. Lett. 2017, 19, 1084–1087. [Google Scholar] [CrossRef]
- Meng, J.; Wen, M.; Zhang, S.; Pan, P.; Yu, X.; Deng, W.P. Unexpected O-H Insertion of Rhodium-Azavinylcarbenes with N-Acylhydrazones: Divergent Synthesis of 3,6-Disubstituted- and 3,5,6-Trisubstituted-1,2,4-Triazines. J. Org. Chem. 2017, 82, 1676–1687. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Obijalska, E.; Kaszyński, P. Access to 1,4-Dihydrobenzo[e][1,2,4]Triazin-4-Yl Derivatives. Org. Lett. 2016, 18, 916–919. [Google Scholar] [CrossRef]
- Abramovitch, R.A.; Schofield, K. Polyazabicyclic Compounds. Part I. Preliminary Experiments on the Bischler and the Bamberger Synthesis of Benzo-1:2:4-Triazines. J. Chem. Soc. 1955, 1955, 2326–2336. [Google Scholar] [CrossRef]
- Reich, M.F.; Fabio, P.F.; Lee, V.J.; Kuck, N.A.; Testa, R.T. Pyrido[3,4-e]-1,2,4-Triazines and Related Heterocycles as Potential Antifungal Agents. J. Med. Chem. 1989, 32, 2474–2485. [Google Scholar] [CrossRef]
- Fort, E.H.; Scott, L.T. Gas-Phase Diels-Alder Cycloaddition of Benzyne to an Aromatic Hydrocarbon Bay Region: Groundwork for the Selective Solvent-Free Growth of Armchair Carbon Nanotubes. Tetrahedron Lett. 2011, 52, 2051–2053. [Google Scholar] [CrossRef]
- Pomikło, D.; Pietrzak, A.; Kishi, R.; Kaszyński, P. Bi-Blatter Diradicals: Convenient Access to Regioisomers with Tunable Electronic and Magnetic Properties. Mater. Chem. Front. 2023, 7, 4928–4943. [Google Scholar] [CrossRef]
- Bodzioch, A.; Pomikło, D.; Celeda, M.; Pietrzak, A.; Kaszyński, P. 3-Substituted Benzo[e][1,2,4]Triazines: Synthesis and Electronic Effects of the C(3) Substituent. J. Org. Chem. 2019, 84, 6377–6394. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.L.; Chen, D.C.; Xie, Z.; Cai, X.; Xie, G.; Liu, K.; Tang, J.; Su, S.J.; Cao, Y. Study of Configuration Differentia and Highly Efficient, Deep-Blue, Organic Light-Emitting Diodes Based on Novel Naphtho[1,2-d]Imidazole Derivatives. Adv. Funct. Mater. 2015, 25, 5190–5198. [Google Scholar] [CrossRef]
- Kokatla, H.P.; Yoo, E.; Salunke, D.B.; Sil, D.; Ng, C.F.; Balakrishna, R.; Malladi, S.S.; Fox, L.M.; David, S.A. Toll-like Receptor-8 Agonistic Activities in C2, C4, and C8 Modified Thiazolo[4,5-c]Quinolines. Org. Biomol. Chem. 2013, 11, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.M.; Kimbrell, M.R.; Malladi, S.S.; David, S.A. Regioisomerism-Dependent TLR7 Agonism and Antagonism in an Imidazoquinoline. Bioorganic Med. Chem. Lett. 2009, 19, 2211–2214. [Google Scholar] [CrossRef]
- Van Galen, P.J.; Nissen, P.; van Wijngaarden, I.; Ijzerman, A.P.; Soudijn, W. 1H-Imidazo [4,5-c] Quinolin-4-Amines: Novel Non-Xanthine Adenosine Antagonists. J. Med. Chem. 1991, 34, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, H.; Ibrahim, M.R.; Al-Awadi, N.A.; Ibrahim, Y.A. Gas-Phase Thermolysis of Condensed-1,2,4-Triazines: Interesting Routes toward Heterocyclic Ring Systems. Tetrahedron 2007, 63, 12948–12953. [Google Scholar] [CrossRef]
- Szamweber, P.; Pietrzak, A.; Zissimou, G.A.; Kaszyński, P. Toward N-Peri-Annulated Planar Blatter Radical through Aza-Pschorr and Photocyclization. J. Org. Chem. 2023, 88, 17197–17205. [Google Scholar] [CrossRef]
- Hodson, H.H.; Walke, J. The Diazotisation of Aromatic Nitro-Amines, Etc. 382. The Diamtisation of Aromatic Nitro-Amines and the Prevention. J. Am. Chem. Soc. 1933, 1620, 1620–1621. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis CCD, CrysAlis RED, CrysAlisPro; Version 1.171.40.84a; Rigaku Oxford Diffraction: Abingdon, UK, 2020. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teymouri, M.; Pietrzak, A.; Bartos, P. (Hetero)Arene Ring-Fused [1,2,4]Triazines. Molbank 2024, 2024, M1824. https://doi.org/10.3390/M1824
Teymouri M, Pietrzak A, Bartos P. (Hetero)Arene Ring-Fused [1,2,4]Triazines. Molbank. 2024; 2024(2):M1824. https://doi.org/10.3390/M1824
Chicago/Turabian StyleTeymouri, Mahshid, Anna Pietrzak, and Paulina Bartos. 2024. "(Hetero)Arene Ring-Fused [1,2,4]Triazines" Molbank 2024, no. 2: M1824. https://doi.org/10.3390/M1824
APA StyleTeymouri, M., Pietrzak, A., & Bartos, P. (2024). (Hetero)Arene Ring-Fused [1,2,4]Triazines. Molbank, 2024(2), M1824. https://doi.org/10.3390/M1824





