Abstract
The previously unknown cyclopropane spiro-fused with isoxazol-5-one ((1RS,3SR)-1-(4-methylbenzyl)-7-phenyl-5-oxa-6-azaspiro[2.4]hept-6-en-4-one) was synthesized from benzylideneisoxazol-5-one in 34% yield via double methylene transfer from diazomethane. The structure of the compound was established based on 1H, 13C, and 2D NMR spectroscopy and high-resolution mass spectrometry, and confirmed by X-ray diffraction analysis.
1. Introduction
The chemistry of donor-acceptor cyclopropanes (DACs) is an actively developing area of research. These reactive strained compounds have been used for the synthesis of carbo- [1,2] and heterocyclic [3,4] molecules including natural compounds [5]. Reliable methods for the synthesis of DACs are available [6], which mainly include the Corey–Chaykovsky reaction and cyclopropanation using diazo compounds.
Most widespread DACs are monocyclic, while DACs spiro-fused with heterocycles are rare. Cyclopropanes spiro-fused with oxindole [7,8,9], imidazolone [10], oxazolone [11], and pyrazolone [12] are known. Several types of cyclopropanes spiro-fused with isoxazol-5-ones were obtained by the Corey–Chaykovsky reaction of benzylideneisoxazol-5-ones with dimethylsulfonium phenacylide [13] or by the reaction of isoxazol-5-ones with benzylidenemalononitrile [14].
In our previous works, we have used different isoxazole derivatives as starting materials for the synthesis of nitrogen heterocycles [15,16,17,18]. In search of new isoxazole substrates, we became interested in isoxazol-5-ones spiro-fused with a cyclopropane ring. Such compounds could be prepared by the cyclopropanation of the C=C bond of 4-benzylideneisoxazol-5-ones. In this work, we report that the reaction of 4-(4-methylbenzylidene)-3-phenylisoxazol-5-one with diazomethane proceeds as a double methylene transfer, affording a benzyl-substituted cyclopropane spiro-fused with isoxazol-5-one. Note that examples of the double methylene transfer are rarely found in the literature [19,20,21,22], and, to the best of our knowledge, have never been observed in the cyclopropane formation.
2. Results and Discussion
Initially, the Corey–Chaykovsky reaction using dimethylsulfoxonium iodide and NaH was tried for the cyclopropanation of (Z)-benzylideneisoxazolone 1 (Scheme 1). However, even at low temperatures, only the tarring of the reaction mixture was observed, and no cyclopropane 2 or other products were detected. Then, we turned to a diazomethane method. Etherial diazomethane solution was prepared from N-nitroso-N-methylurea and KOH pellets at 0 °C and added dropwise to a solution of benzylideneisoxazolone 1. To our delight, several products were observed according to TLC. The reaction mixture was subjected to flash chromatography on silica gel, and a major product 3 comprising two new methylene groups was isolated. Additional recrystallization from the Et2O–hexane mixture afforded compound 3 in 34% yield in pure form as a single (1RS,3SR)-diastereomer. The structure of compound 3 was established on the basis of 1H, 13C, and 2D NMR spectra, as well as HRMS.
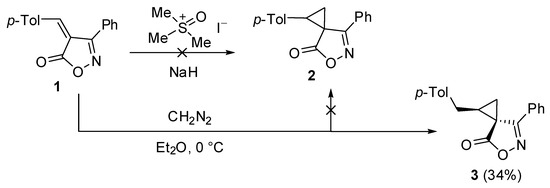
Scheme 1.
Synthesis of spirocyclopropane 3.
Finally, the structure and stereochemistry of product 3 were confirmed by monocrystal X-ray diffraction analysis (Figure 1). In the cyclopropane ring, a benzyl substituent is trans-oriented to a phenyl substituent of the isoxazolone.
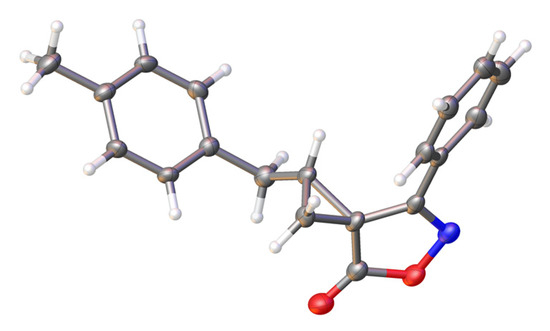
Figure 1.
Molecular structure of spirocyclopropane 3; thermal ellipsoids are drawn at a 50% probability level.
The possible mechanism explaining the formation of cyclopropane 3 is depicted in Scheme 2. We assume that, initially, tolyl-substituted cyclopropane 2 is formed. Probably, it is unstable, and readily suffers the C–C bond cleavage to form 1,3-dipole 4 due to the effective stabilization of both a cationic center by a tolyl substituent and an anionic center in the isoxazole cycle. Further hydride shift leads to ethylideneisoxazol-5-one 5, which, in turn, undergoes cyclopropanation yielding the relatively stable benzyl-substituted cyclopropane 3.
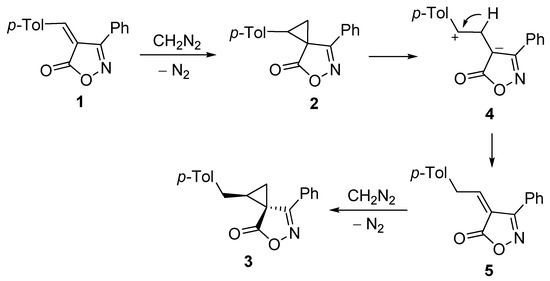
Scheme 2.
Proposed mechanism for the formation of spirocyclopropane 3.
3. Materials and Methods
3.1. General Instrumentation
The melting point was determined on a Stuart SMP30 melting-point apparatus. NMR spectra were recorded on a Bruker Avance 400 spectrometer in CDCl3. 1H and 13C{1H} NMR spectra were calibrated according to the residual signal of CDCl3 (δ = 7.26 ppm) and the carbon atom signal of CDCl3 (δ = 77.0 ppm), respectively. High-resolution mass spectra were recorded with a Bruker maXis HRMS-QTOF, via electrospray ionization. Thin-layer chromatography (TLC) was conducted on aluminum sheets precoated with SiO2 ALUGRAM SIL G/UV254. Column chromatography was performed on silica gel 60 M (0.04–0.063 mm). Diethyl ether was distilled over sodium metal and stored over it. 4-(4-Methylbenzyl)-3-phenylisoxazol-5(4H)-one 1 was prepared using the reported procedure [23].
Single crystals of compound 3 were grown by the slow evaporation of its solution in diethyl ether–hexane mixture. Crystallographic data were collected on a SuperNova, single source at offset/far, HyPix3000 diffractometer using graphite monochromatic Cu–Kα radiation (λ = 1.54184 Å). The crystal was kept at 99.97(16) K during data collection. Using the Olex2 [24], the structure was solved with the ShelXT [25] structure solution program using the Intrinsic Phasing method and refined with the ShelXL [26] refinement package using Least Squares minimization. CCDC 2330024 contains crystallographic data for compound 3. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
3.2. (1RS,3SR)-1-(4-Methylbenzyl)-7-phenyl-5-oxa-6-azaspiro[2.4]hept-6-en-4-one (3)
To a suspension of isoxazolone 1 (0.900 mmol, 237 mg) in diethyl ether (0.3 M, 3 mL), a large excess of the ~0.3 M solution of CH2N2 in diethyl ether (30 mL), prepared from N-nitroso-N-methylurea and KOH pellets at 0 °C (CAUTION! Diazomethane is carcinogenic and potentially explosive), was added dropwise over 20 min. After full consumption of the starting material (checked by TLC), the reaction was quenched with 10% aqueous acetic acid and extracted with ethyl acetate. Combined organic layers were washed with water and brine, and dried over Na2SO4. Evaporation of the solvent and flash column chromatography (eluent petroleum ether–ethyl acetate, 5:1) followed by subsequent recrystallization from the diethyl ether–hexane mixture gave 89 mg (34%) of product 3.
Mp: 110–112 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.55–7.51 (m, 1H), 7.48–7.44 (m, 4H), 7.14 (d, J = 7.9 Hz, 2H), 7.08 (d, J = 7.9 Hz, 2H), 3.16 (qd, J = 14.9, 7.1 Hz, 2H), 2.49–2.42 (m, 1H), 2.37–2.34 (m, 1H), 2.34 (s, 3H), 2.04 (dd, J = 8.8, 5.1 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 177.3, 167.0, 136.3, 135.8, 131.3, 129.5, 129.2, 128.2, 127.0, 126.8, 35.4, 31.8, 30.8, 26.2, 21.0. HRMS (ESI-TOF) calculated for C19H18NO2 [M + H]+ 292.1332; found 292.1330.
4. Conclusions
The previously unknown cyclopropane spiro-fused with isoxazol-5-one ((1RS,3SR)-1-(4-methylbenzyl)-7-phenyl-5-oxa-6-azaspiro[2.4]hept-6-en-4-one) was synthesized from benzylideneisoxazol-5-one in 34% yield via double methylene transfer from diazomethane. The structure of the compound was established based on NMR spectroscopy and high-resolution mass spectrometry, and confirmed by X-ray diffraction analysis.
Supplementary Materials
The following supporting information can be downloaded online. 1H, 13C{1H}, 2D NMR spectra of compound 3; crystallographic data for compound 3.
Author Contributions
Conceptualization, G.D.T. and N.V.R.; methodology, G.D.T. and N.V.R.; investigation, G.D.T.; writing—original draft preparation, G.D.T. and N.V.R.; writing—review and editing, G.D.T. and N.V.R.; supervision, N.V.R.; project administration, N.V.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (Grant No. 22-73-10184).
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
Dedicated to the 300th anniversary of St. Petersburg State University. This research used resources of the Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Cryogenic Centre, and Centre for X-ray Diffraction Studies of the Research Park of St. Petersburg State University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grover, H.K.; Emmett, M.R.; Kerr, M.A. Carbocycles from donor-acceptor cyclopropanes. Org. Biomol. Chem. 2015, 13, 655–671. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Trushkov, I.V. Donor–acceptor cyclopropanes in the synthesis of carbocycles. Chem. Rec. 2019, 19, 2189–2208. [Google Scholar] [CrossRef]
- Singh, P.; Varshnaya, R.K.; Dey, R.; Banerjee, P. Donor-Acceptor Cyclopropanes as an Expedient Building Block Towards the Construction of Nitrogen-Containing Molecules: An Update. Adv. Synth. Catal. 2020, 362, 1447–1484. [Google Scholar] [CrossRef]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A New Golden Age for Donor-Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Nanda, L.N. Application of oxygen/nitrogen substituted donor-acceptor cyclopropanes in the total synthesis of natural products. Tetrahedron Lett. 2017, 58, 711–720. [Google Scholar] [CrossRef]
- Tomilov, Y.V.; Menchikov, L.G.; Novikov, R.A.; Ivanova, O.A.; Trushkov, I.V. Methods for the synthesis of donor-acceptor cyclopropanes. Russ. Chem. Rev. 2018, 87, 201–250. [Google Scholar] [CrossRef]
- Zaytsev, S.V.; Ivanov, K.L.; Skvortsov, D.A.; Bezzubov, S.I.; Melnikov, M.Y.; Budynina, E.M. Nucleophilic Ring Opening of Donor-Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-pxindoles]. J. Org. Chem. 2018, 83, 8695–8709. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, Q.; Chen, L.; Deng, H.; Cai, H.; Zhang, Q. Lewis-acid-catalyzed [3+2] annulation of oxindole based spirocyclic donor-acceptor cyclopropanes with ynamides. Org. Biomol. Chem. 2021, 19, 9645–9648. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, F.; Yu, Y.; Zhou, J. A Highly Diastereo- and Enantioselective Hg(II)-Catalyzed Cyclopropanation of Diazooxindoles and Alkenes. Org. Lett. 2013, 15, 42–45. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Kuleshov, A.V.; Solyev, P.N.; Korlyukov, A.A.; Dorovatovskii, P.V.; Mineev, K.S.; Baranov, M.S. Imidazol-5-one as an Acceptor in Donor–Acceptor Cyclopropanes: Cycloaddition with Aldehydes. Org. Lett. 2020, 22, 2740–2745. [Google Scholar] [CrossRef]
- Arenal, I.; Bernabe, M.; Fernandez-Alvarez, E.; Penades, S. Synthesis of (E)- and (Z)-1-Amino-2-aryl(methyl)-cyclopropanecarboxylic Acid via Spirooxazolones. Synthesis 1985, 8, 773–775. [Google Scholar] [CrossRef]
- Mustafa, A.; Asker, W.; Harhash, A.H.; Fleifel, A.M. Reactivity of unsaturated centres in heterocycles and chalkones toward diazoalkanes. Tetrahedron 1965, 21, 2215–2229. [Google Scholar] [CrossRef]
- Croce, P.D. Heterocycles from ylides. Part V (1). Reactivity of 4-arylmethylene-Δ2-isoxazolin-5-ones with stabilized sulfur ylides. J. Heterocycl. Chem. 1976, 13, 1109–1110. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Korshunov, A.D.; Anisina, Y.E.; Novikov, R.A.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Stereoselective Michael Halogenation Initiated Ring Closure (MHIRC) Synthesis of Spirocyclopropanes from Benzylidenemalononitriles and 3-Arylisoxazol-5(4H)-ones. Synlett 2016, 27, 2489–2493. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, N.; Kumar, R.; Banerjee, P. α,β-Unsaturated Carbonyls for One Pot Transition-Metal-Free Access to 3,6-Dihydro-2H-pyrans. J. Org. Chem. 2022, 87, 7167–7178. [Google Scholar] [CrossRef]
- Senatore, R.; Malik, M.; Langer, T.; Holzer, W.; Pace, V. Consecutive and Selective Double Methylene Insertion of Lithium Carbenoids to Isothiocyanates: A Direct Assembly of Four-Membered Sulfur-Containing Cycles. Angew. Chem. Int. Ed. 2021, 60, 24854–24858. [Google Scholar] [CrossRef]
- Butova, E.D.; Barabash, A.V.; Petrova, A.A.; Kleiner, C.M.; Schreiner, P.R.; Fokin, A.A. Stereospecific Consecutive Epoxide Ring Expansion with Dimethylsulfoxonium Methylide. J. Org. Chem. 2010, 75, 6229–6235. [Google Scholar] [CrossRef]
- Mukherjee, P.; Pettersson, M.; Dutra, J.K.; Xie, L.; Ende, C.W. Trifluoromethyl Oxetanes: Synthesis and Evaluation as a tert-Butyl Isostere. ChemMedChem 2017, 12, 1574–1577. [Google Scholar] [CrossRef]
- Vasilchenko, D.S.; Agafonova, A.V.; Simdianov, I.V.; Koronatov, A.N.; Sakharov, P.A.; Romanenko, I.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. 2H-1,2,3-triazole-4-carboxylic acids via Ru(II)-catalyzed rearrangement of 4-hydrazonoisoxazol-5-ones. Tetrahedron Lett. 2023, 123, 154580. [Google Scholar] [CrossRef]
- Filippov, I.P.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V. One-Pot Synthesis of Multifunctionalized 1-Pyrrolines from 2-Alkyl-2H-azirines and Diazocarbonyl Compounds. J. Org. Chem. 2022, 87, 8835–8840. [Google Scholar] [CrossRef]
- Tiuftiakov, N.Y.; Strelnikova, J.O.; Filippov, I.P.; Khaidarov, A.R.; Khlebnikov, A.F.; Bunev, A.S.; Novikov, M.S.; Rostovskii, N.V. Rhodium-Catalyzed Synthesis of 2-Aroylpyrimidines via Cascade Heteropolyene Rearrangement. Org. Lett. 2021, 23, 6998–7002. [Google Scholar] [CrossRef]
- Titov, G.D.; Antonychev, G.I.; Novikov, M.S.; Khlebnikov, A.F.; Rogacheva, E.V.; Kraeva, L.A.; Rostovskii, N.V. Gold vs Light: Chemodivergent Reactivity of Diazoesters toward 2H-Azirine-2-carboxylic Acids. Org. Lett. 2023, 25, 2707–2712. [Google Scholar] [CrossRef]
- Dias-Jurberg, I.; Gagosz, F.; Zard, S.Z. Unusual Approach to Branched 3-Alkynylamides and to 1,5-Dihydropyrrol-2-ones. Org. Lett. 2010, 12, 416–419. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).