(E)-1-(4-Methoxyphenyl)-5-methyl-4-(1-phenyl-4-((2-(2,4,6-trichlorophenyl)hydrazineylidene)methyl)-1H-pyrazol-3-yl)-1H-1,2,3-triazole
Abstract
1. Introduction
2. Results and Discussion
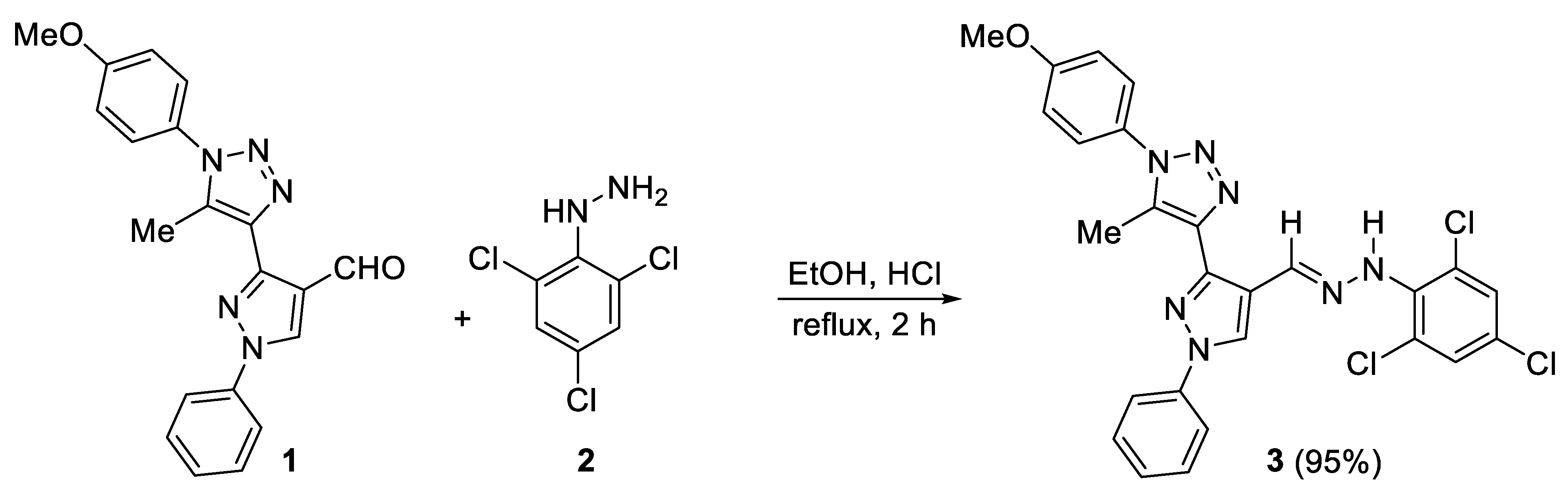
2.1. Synthesis of 3
2.2. IR and NMR Spectroscopy of 3
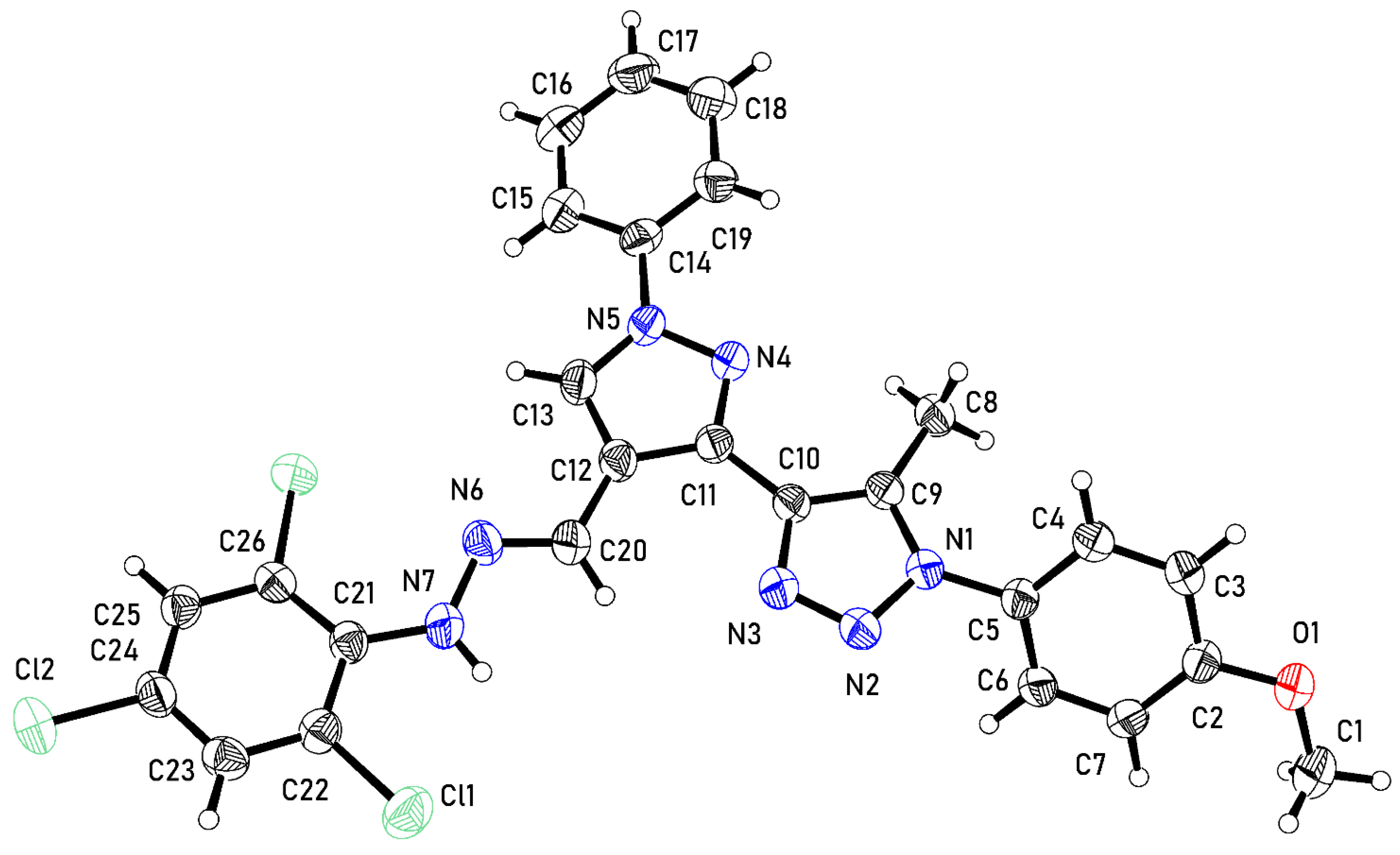
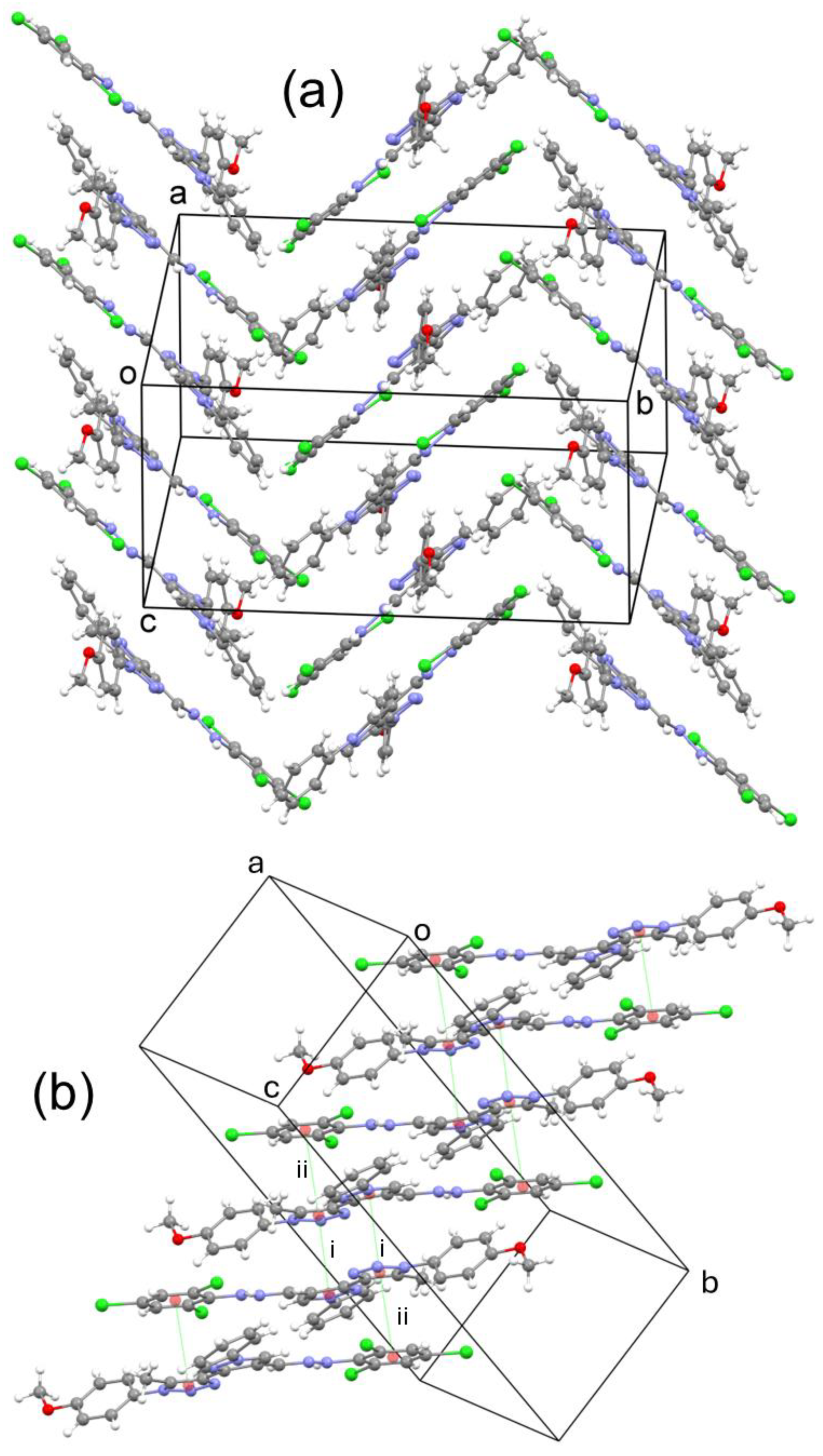
2.3. Crystal Structure of 3
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-containing compounds as anti-lung cancer agents: Current developments, mechanisms of action, and structure-activity relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yan, H.; Wang, J.; Liu, K.; Liu, B.; Shi, Y. 1,2,3-Triazole-containing hybrids with potential antibacterial activity against ESKAPE pathogens. Eur. J. Med. Chem. 2022, 244, 114888. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, e2100158. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A. Pyrazole: An emerging privileged scaffold in drug discovery. Future Med. Chem. 2023, 15, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Turones, L.C.; Martins, A.N.; Moreira, L.K.D.S.; Fajemiroye, J.O.; Costa, E.A. Development of pyrazole derivatives in the management of inflammation. Fundam. Clin. Pharmacol. 2021, 35, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.; Khatri, M.; Punia, R.; Sindhu, S. Recent progress in anticancer agents incorporating pyrazole scaffold. Mini Rev. Med. Chem. 2022, 22, 115–163. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.; Sharma, D.K. Pyrazole; a privileged scaffold of medicinal chemistry: A comprehensive review. Curr. Top. Med. Chem. 2023, 23, 2097–2115. [Google Scholar] [CrossRef]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied. Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Carneiro Brum, J.; França, T.C.C.; LaPlante, S.R.; Villar, J.D.F. Synthesis and biological activity of hydrazones and derivatives: A review. Mini Rev. Med. Chem. 2020, 20, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jamwal, P.; Vaid, H.; Gurubrahamam, R. Synthesis of alkynyl hydrazones from unprotected hydrazine and their reactivity as diazo precursors. Org. Lett. 2023, 11, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Xiong, H. Recent developments in 1,2,3-triazole-based chemosensors. Dyes Pigm. 2021, 185, 108905. [Google Scholar] [CrossRef]
- Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.; Kiatkamjornwong, S. Furan/thiophene-based fluorescent hydrazones as fluoride and cyanide sensors. J. Photochem. Photobiol. A Chem. 2019, 385, 112038. [Google Scholar] [CrossRef]
- Aysha, T.S.; Mohamed, M.B.I.; El-Sedik, M.S.; Youssef, Y.A. Multi-functional colorimetric chemosensor for naked eye recognition of Cu2+, Zn2+ and Co2+ using new hybrid azo-pyrazole/pyrrolinone ester hydrazone dye. Dyes Pigm. 2021, 196, 109795. [Google Scholar] [CrossRef]
- Govindasamy, V.; Perumal, S.; Sekar, I.; Madheswaran, B.; Karuppannan, S.; Kuppannan, S.B. Phenothia-zine-thiophene hydrazide dyad: An efficient “on-off” chemosensor for highly selective and sensitive detection of Hg2+ ions. J. Fluoresc. 2021, 31, 667–674. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent sensors for the detection of heavy metal ions in aqueous media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, L.; Liu, F.; Fan, Y.; Liu, Y.; Han, Y.; Hu, Y.; Su, J.; Song, C. Rapid and selective detection of aluminum ion using 1,2,3-triazole-4,5-dicarboxylic acid-functionalized gold nanoparticle-based colorimetric sensor. RSC Adv. 2021, 11, 30635–30645. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S.C3H7NO. Z. Kristallogr. New Cryst. Struct. 2020, 235, 915–917. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Kariuki, B.M.; Mohamed, H.A.; Bekheit, M.S.; Awad, H.M.; El-Hiti, G.A. Synthesis and anticancer activity of 3-(1-aryl-5-methyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes. J. Mol. Struct. 2023, 1294, 136528. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Khidre, R.E.; Mohamed, H.A.; El-Hiti, G.A. A simple process for the synthesis of novel pyrazolyltriazole and dihydropyrazolylthiazole derivatives as antimicrobial agents. Arab. J. Sci. Eng. 2017, 42, 2441–2448. [Google Scholar] [CrossRef]
- Zhuang, W.; Saaby, S.; Jorgensen, K.A. Direct organocatalytic enantioselective Mannich reactions of ketimines: An approach to optically active quaternary alpha-amino acid derivatives. Angew. Chem. Int. Ed. Engl. 2004, 43, 4476–4478. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Li, D.F.; Sun, J.; Gao, J.H.; Shan, S. (E)-Benzaldehyde (2,4,6-trichlorophenyl)hydrazone. Acta Cryst. 2011, E67, o528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shang, Z.-R.; Li, X.-T.; Zhang, J.-N.; Wang, Y.; Li, K.; Li, Y.-Y.; Zhang, Z.-H. Simple and efficient approach for synthesis of hydrazones from carbonyl compounds and hydrazides catalyzed by meglumine. Synth. Commun. 2016, 47, 178–187. [Google Scholar] [CrossRef]
- Ashok, D.; Ram, R.M.; Nagaraju, N.; Dharavath, R.; Ramakrishna, K.; Gundu, S.; Shravani, P.; Sarasija, M. Microwave-assisted synthesis and in-vitro antiproliferative activity of some novel 1,2,3-triazole-based pyrazole aldehydes and their benzimidazole derivatives. Med. Chem. Res. 2020, 29, 699–706. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wahab, B.F.; Mohamed, H.A.; Kariuki, B.M.; El-Hiti, G.A. (E)-1-(4-Methoxyphenyl)-5-methyl-4-(1-phenyl-4-((2-(2,4,6-trichlorophenyl)hydrazineylidene)methyl)-1H-pyrazol-3-yl)-1H-1,2,3-triazole. Molbank 2024, 2024, M1798. https://doi.org/10.3390/M1798
Abdel-Wahab BF, Mohamed HA, Kariuki BM, El-Hiti GA. (E)-1-(4-Methoxyphenyl)-5-methyl-4-(1-phenyl-4-((2-(2,4,6-trichlorophenyl)hydrazineylidene)methyl)-1H-pyrazol-3-yl)-1H-1,2,3-triazole. Molbank. 2024; 2024(2):M1798. https://doi.org/10.3390/M1798
Chicago/Turabian StyleAbdel-Wahab, Bakr F., Hanan A. Mohamed, Benson M. Kariuki, and Gamal A. El-Hiti. 2024. "(E)-1-(4-Methoxyphenyl)-5-methyl-4-(1-phenyl-4-((2-(2,4,6-trichlorophenyl)hydrazineylidene)methyl)-1H-pyrazol-3-yl)-1H-1,2,3-triazole" Molbank 2024, no. 2: M1798. https://doi.org/10.3390/M1798
APA StyleAbdel-Wahab, B. F., Mohamed, H. A., Kariuki, B. M., & El-Hiti, G. A. (2024). (E)-1-(4-Methoxyphenyl)-5-methyl-4-(1-phenyl-4-((2-(2,4,6-trichlorophenyl)hydrazineylidene)methyl)-1H-pyrazol-3-yl)-1H-1,2,3-triazole. Molbank, 2024(2), M1798. https://doi.org/10.3390/M1798






