Abstract
The reaction of the dianionic species [Fe2(CO)6(μ-PPh)2]2− with tBuN(CH2Cl)2 gives the di-iron carbonyl aza-diphosphido-bridged complex [Fe2(CO)6(µ-{P(Ph)CH2}2NtBu)] (1). Attempts to prepare 1 by click-chemistry by reacting [Fe2(CO)6(μ-PHPh)2] with CH2O and tBuNH2 afforded a bis-phosphido compound [Fe2(CO)6(µ-P(Ph)CH2NHtBu)2] (2) which exists as two, syn and anti, isolable isomers depending on the relative orientation of the groups carried by the phosphorus atoms. In the presence of HBF4.Et2O, in dichloromethane, 1 leads to the stabilized ammonium species [Fe2(CO)6(µ-{P(Ph)CH2}2NHtBu)](BF4) (3). The derivatives 1–3 were characterized by IR and 1H, 31P-{1H} NMR spectroscopies. Their structures in a solid state were determined by X-ray diffraction analyses, which accord with their spectroscopic characteristics.
1. Introduction
In recent decades, the chemistry of carbonyl iron complexes has undergone intensive development within the field of biomimicry. Alongside numerous reports on carbonyl dithiolato-bridged di-iron complexes structurally close to the active site of [FeFe]-hydrogenases (H-cluster) (Scheme 1a) [1,2,3,4,5], phosphido-bridged analogues (Scheme 1b,c) were studied in order to compare the electronic and stereochemical effects of the replacement of the µ-S2R bridge with the µ-PR2 group on the reactivity and redox properties of such carbonyl di-iron systems [6,7,8,9,10,11,12,13,14,15,16,17,18,19]. The chemistry of bis-phosphido-bridged di-iron complexes of general formula [Fe2(CO)6(µ-PR2)2] and linked-diphosphido [Fe2(CO)6(µ-(PR)2R’)] dates back much earlier [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Electrochemical and theoretical calculations on robust complexes [Fe2(CO)6(µ-PRR’)(µ-PRR″)] were carried out because most of these compounds present a reversible two-electron reduction involving a potential inversion [15,16,44] depending on R, R’ and R″ groups which affect Fe-P distances and the geometry of the {Fe2P2} core. Among these complexes with a {Fe2P2} core, diphosphido compounds featuring a µ-η2:η2-(RP-CH2-R’-CH2-PR″) bridge were more recently the subject of interest (Scheme 1c) [11,12,13,14,15,16,17]. To the best of our knowledge, the compound [Fe2(CO)6(µ-{P(Ph)CH2}2N(CH2)2OCH3] (Scheme 1d) is the sole example of an azadiphosphido-bridged complex [11]. We report herein an extension of our work for preparing such scarce µ-aza-diphosphido complexes. The syntheses, spectroscopic and structural characterizations of the complex [Fe2(CO)6(µ-{P(Ph)CH2}2NtBu)] (1), its protonated form (3) and a bis-phosphido side-product [Fe2(CO)6(µ-P(Ph)CH2NHtBu)2] (2) are presented.
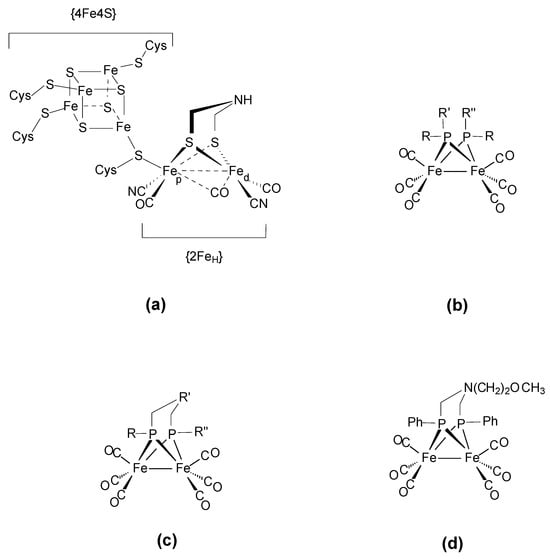
Scheme 1.
Representations of (a) the H-cluster, (b) carbonyl di-iron bis-phosphido complexes, (c) carbonyl diphosphido complexes and (d) aza-diphosphido complexes.
2. Results and Discussion
The complex [Fe2(CO)6(µ-{P(Ph)CH2}2NtBu)] (1) was obtained from the reaction of tBuN(CH2Cl)2 with the dianion [Fe2(CO)6(µ-PPh)2]2− (Scheme 2, path (a)). This latter dianion was prepared by the reaction of [Fe2(CO)6(µ-PHPh)2] with two equivalents of methyllithium [21,31,41]. In the presence of 1 equivalent of HBF4, Et2O, the protonation of 1 occurred at the nitrogen atom of the azadiphosphido bridge, which produced the cationic complex [Fe2(CO)6(µ-{P(Ph)CH2}2NHtBu)](BF4) (3). Attempts to prepare 1 through a one-pot reaction following a click process were also performed (Scheme 2, path (b)). The two isomers 2-anti and 2-syn were isolated by reacting a solution of the complex [Fe2(CO)6(µ-PHPh)2] in ethanol with an aqueous solution of CH2O, followed by the addition of tBuNH2. Compounds 1–3 were characterized by spectroscopic analyses (IR, NMR), and their structures were confirmed by X-ray analyses of single crystals (see Appendix A and Tables S1–S3). The strong bands observed at 2053, 2013, 1984 and 1970 cm−1 in the carbonyl region of the infrared spectrum of a CH2Cl2 solution of 1 are typical of hexacarbonyl diiron (FeIFeI) complexes with a diphosphido bridge [11]. The protonation at the amine function in 1, giving the ammonium species 3, is revealed by a shift of ca 12 cm−1 (average) of the ν(CO) bands at higher wavenumbers compared to those of 1, which is typical of a protonation in the second sphere of coordination of the di-iron centre. It is worth noting that few examples of isolated N-protonated forms of thiolato analogues have been reported [2,5]. The IR spectra of 2-syn/anti are similar to that of 1. The 1H NMR spectrum of 1 in CDCl3 exhibits the expected set of resonances for the aza-diphosphido bridge {P(Ph)CH2}2NtBu} according to a symmetrical molecule. Similar signals were observed in the 1H NMR spectrum of 3 in CD3CN, but shifted at higher chemical shifts due to its cationic nature. The proton of the ammonium group could not be observed. The 1H NMR spectra of 2-syn/anti in CDCl3 differ from those of 1 and 3 in the tBu/CH2/C6H5 ratio, which shows that there are two tBu groups instead of one. In the case of the syn isomer, a singlet is observed for the tBu groups, while the 1H NMR spectrum of the anti isomer displays two inequivalent singlets. Symmetrical species 1, 2-syn, 3 present in their 31P-{1H} NMR spectra a singlet above 110 ppm. Interestingly, in the case of the 2-anti species, an AB pattern with a coupling constant of 130 Hz was detected at 147.7 ppm. These chemical shifts are in the range of those observed when phosphido groups bridge two metal centres connected by a metal–metal interaction [46].
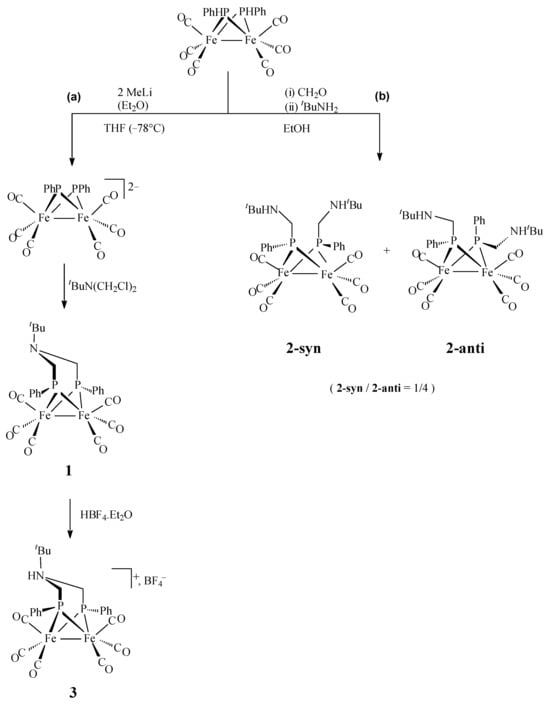
Scheme 2.
Synthetic pathways for complexes 1–3: (a) Sequential steps pathway and (b) one-pot pathway.
Single crystals suitable for X-ray diffraction studies were obtained, at −15 °C, from CH2Cl2: Et2O or EtOH: Et2O solutions for compounds 1, 3 and 2, respectively (Figure 1 and Table 1). The overall geometry of the three complexes 1–3 is similar to those of analogous compounds with a butterfly {Fe2P2} core, two eclipsed square-pyramidal {Fe(CO)3} moieties bridged by two phosphido groups and an Fe—Fe interaction [11]. The distances of the Fe-Fe bond in 1 and 3, 2.6361(5) Å and 2.6242(8) Å, respectively, are close to that observed in [Fe2(CO)6(µ-{P(Ph)CH2}2N(CH2)2OMe)] (2.631(1) Å), while such a distance is much longer in 2 (2.6693(5) Å) (Table 1). The intramolecular P1···P2 contact is significantly shorter in complexes 1 and 3 (2.6880(9) Å and 2.6816(14) Å, respectively)] relative to that determined in 2-anti (2.8899 Å) due to the steric constraint imposed by the aza-diphosphido bridge. The Fe2—P2—C8—N1—C7—P1 metalloheterocycle in 1 and 3 adopts a chair conformation with the tBu substituent in an equatorial position. The geometry around N1 in 1 is a nearly trigonal pyramid (see C8—N1—C7, C8—N1—C9, C7—N1—C9 angles in Table 1). Indirect hints of the N-protonation in 3 are revealed by the opening of the Fe(1)—Fe(2)—C(1) angle of 7° to minimize H/CO steric clash (Fe1—Fe2—C1 = 152.48(16)° in 3 and 144.79(9)° in 1) and by a slight lengthening of N-C bonds in 3 compared with those in 1 (Table 1). In addition, the N—H proton is involved in a stabilizing hydrogen bond interaction to a hydrous H2O…F-BF3− anion [d(N1-H1…O1W) = 2.7056(1) Å, (N1—H1—O1W) = 172.418(4)°, d(F4…O1W) = 2.7081(1) Å, (F4—H1W—O1W) = 160.799(4)°].
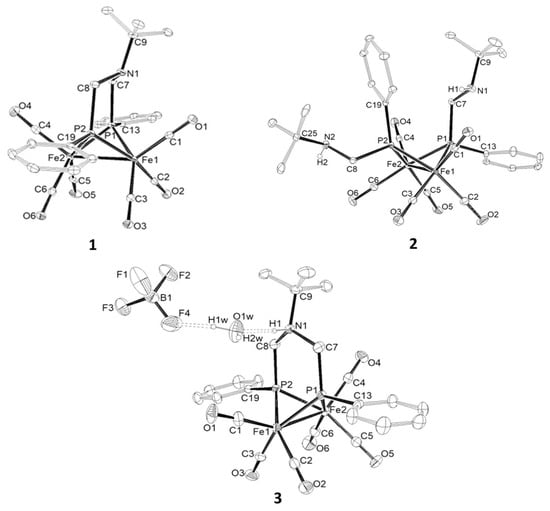
Figure 1.
Molecular structure of compounds 1–3 with thermal ellipsoids at 20% probability.

Table 1.
Selected bond lengths (Å) and angles (°) of 1–3.
3. Materials and Methods
All the experiments were carried out under an inert atmosphere, using Schlenk techniques for the syntheses. Solvents were deoxygenated and dried according to standard procedures. A literature method was used for the preparation of the starting compound [Fe2(CO)6(μ-PHPh)2] [47] and tBuN(CH2Cl)2 [48]. All other reagents were commercially available and used as purchased. NMR spectra (1H, 31P-{1H}) were recorded at room temperature with Bruker AMX 400 or AC 300 spectrometers (Bruker, Billerica, MA, USA) of the “Service général des plateformes, Université de Bretagne Occidentale, Brest” and were referenced to SiMe4 (1H) and H3PO4 (31P). The infrared spectra were recorded with Bruker Vertex 70 and FT IR Perkin Elmer spectrum 2 spectrometer (PerkinElmer Inc., Waltham, MA, USA). Crystal data of compounds 1–3 were collected with an Oxford Diffraction X—Calibur −2 CCD diffractometer (Agilent Technologies Inc., Santa Clara, CA, USA), equipped with a jet cooler device and graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The structures were solved and refined using standard procedures [49]. Deposition numbers CCDC 2330851, 2330852 and 2330853 contain the supplementary crystallographic data for 1, 2 and 3. These data can also be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 4 February 2024).
4. Conclusions
These results show the feasibility of extending the preparation of aza-diphosphido complexes [Fe2(CO)6(µ-{P(Ph)CH2}2NR)] with a steric crowding amine function (R = tBu) through a synthetic pathway involving the handy precursor [Fe2(CO)6(μ-PHPh)2]. The protonation of 1 is directed to the amine site, cleanly affording its stable N-protonated form. X-ray study of the ammonium complex 3 reveals an interesting structural feature with stabilizing hydrogen interactions {F3BF…H2O…HN} involving a water molecule between the counter-anion BF4− and the ammonium function. The one-pot reaction of [Fe2(CO)6(μ-PHPh)2] in the presence of CH2O and tBuNH2 turned out differently and afforded the separable anti and syn isomers of the bis-phosphido compound [Fe2(CO)6(µ-P(Ph)CH2NHtBu)2] (2) with ‘PCH2NHtBu’ linkages. The intermediate formation of the hydroxo species [Fe2(CO)6{μ-PPh(CH2OH)}2] is involved in this reaction. Its preparation with the aim of investigating its reactivity towards amine should help to understand and improve this click process.
Supplementary Materials
Table S1: Crystal data and structure refinement for complex 1; Table S2: Crystal data and structure refinement for complex 2; Table S3: Crystal data and structure refinement for complex 3; Figure S1: IR spectrum in CH2Cl2 of 1; Figure S2: 1H NMR spectrum (CDCl3) of 1; Figure S3: 31P-{1H} NMR spectrum (CDCl3) of 1; Figure S4: IR spectrum in CH2Cl2 of 2-anti; Figure S5: 1H NMR spectrum (CDCl3) of 2-anti; Figure S6: 31P-{1H} NMR spectrum (CDCl3) of 2-anti; Figure S7: IR spectrum in CH2Cl2 of 2-syn; Figure S8: 1H NMR spectrum (CDCl3) of 2-syn; Figure S9: 31P-{1H} NMR spectrum (CDCl3) of 2-syn; Figure S10: IR spectrum in CH2Cl2 of 3; Figure S11: 31P-{1H} NMR spectrum (CDCl3) of 3; Figure S12: 1H NMR spectrum (CDCl3) of 3.
Author Contributions
P.S. and P.D. supervised the syntheses and X-ray, spectroscopic characterizations of the compounds 1–3. C.E., F.Y.P., P.D. and P.S. contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
CNRS (Centre National de la Recherche Scientifique) and the Université de Bretagne Occidentale are acknowledged for financial support.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank K.W. Muir and the University of Glasgow for providing crystallographic measurements of 1–3. We are grateful to F. Michaud for his help with the resolution of X-ray structures.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Preparation of [Fe2(CO)6(µ-{P(Ph)CH2}2NtBu)] (1). A THF solution (50 mL) of Li2[Fe2(CO)6(μ-PPh)2], generated in situ (30 min stirring), at −78 °C, from [Fe2(CO)6(μ-PHPh)2] (0.4 g, 0.80 mmol) with 2 equiv (1.6 mmol) of MeLi (1.1 mL, 1.5 M in Et2O) was treated with a THF solution of tBuN(CH2Cl)2 (0.15 g, 0.88 mmol). After 30 mn, the orange-brown solution was allowed to warm to room temperature and stirred for 1h. The solvent was then removed, and the crude product was extracted with dichloromethane (2 × 25 mL). The extracts were evaporated to dryness, and the residue was chromatographed on a silica gel column. Elution with hexane-dichloromethane (1:1) afforded a bright yellow solution of 1, which was evaporated under vacuum. Yellow crystals grew from CH2Cl2-Et2O (3:1) mixtures, yield 0.196 g (41%). Elemental analysis (%) calculated for C24H23Fe2NO6P2: C 48.44, H 3.89, N 2.35; found; C 46.46, H 4.18, N 1.42. Elemental analysis (%) calculated for 1. ½CH2Cl2 C24.5H24ClFe2NO6P2: C 46.15, H 3.79, N 2.19. 1H NMR (CDCl3, 25 °C): δ = 7.72–7.47 (m, 10H, C6H5), 2.92 (s, 4H, P-CH2-N), 0.97 (s, 9H, N-C(CH3)3); 31P-{1H} NMR (CDCl3, 25 °C): δ = 141.79 (s); IR (CH2Cl2): ν(CO) = 2053(s), 2013(vs), 1984(s), 1970(s) cm−1.
Reaction of [Fe2(CO)6(μ-PHPh)2] with CH2O and tBuNH2. A solution of [Fe2(CO)6(μ-PHPh)2] (0.2 g, 0.40 mmol) in 30 mL of ethanol was treated with 0.1 mL (1.23 mmol) of an aqueous solution of CH2O (37%). After stirring for 30 min at room temperature, 0.5 mL of tBuNH2 (4.8 mmol) was added to the resulting orange-red solution. After the reaction mixture turned brown, the solvent was evaporated to dryness and the crude product was extracted with 3 × 15 mL of Et2O. After filtration and evaporation of Et2O, the resulting residue was chromatographed on a silica gel column with mixture of hexane-CH2Cl2. Elution with dichloromethane afforded a yellow fraction of anti-[Fe2(CO)6(µ-P(Ph)CH2NtBu)2] (2-anti) as the major product of the reaction. A second yellow fraction of the minor isomer syn-[Fe2(CO)6(µ-P(Ph)CH2NHtBu)2] (2-syn) was collected with a mixture of solvents CH2Cl2-THF (9:1).
Yields: 2-anti: 136 mg (51%); 2-syn: 34 mg (13%). Single crystals of 2-anti grew from EtOH-Et2O (1:1) mixtures.
2-anti: Elemental analysis (%) calculated for C28H34Fe2N2O6P2: C 50.33, H 5.13, N 4.19; found; C 50.22, H 5.13, N 4.03. 1H NMR (CDCl3, 25 °C): δ = 7.71–7.35 (m, 10H, C6H5), 3.38 (s, 4H, P-CH2-N), 0.82 (s, 9H, C(CH3)3), 0.36 (s, 9H, C(CH3)3), NH not assigned; 31P-{1H} NMR (CDCl3, 25 °C): δ = 147.7 (AB, JPP = 130.0 Hz); IR (CH2Cl2): ν(CO) = 2051(s), 2012(vs), 1981(s), 1964(s) cm−1.
2-syn: Elemental analysis (%) calculated for C28H34Fe2N2O6P2: C 50.33, H 5.13, N 4.19; found; C 49.61, H 5.11, N 3.89. 1H NMR (CDCl3, 25 °C): δ = 7.10–6.77 (m, 10H, C6H5), 3.33 (s, 4H, P-CH2-N), 0.78 (s, 18H, C(CH3)3), NH not assigned; 31P-{1H} NMR (CDCl3, 25 °C): δ = 136.8 (s); IR (CH2Cl2): ν(CO) = 2050(s), 2011(vs), 1979(s), 1964(s) cm−1.
Protonation of 1. A solution of 1 (0.1 g, 0.17 mmol) in dichloromethane (10 mL) was treated with 1 equiv (23 µL) of HBF4. Et2O (1.19 g/mL). The mixture was stirred for 1 h. The volume was then reduced under vacuum, and diethyl ether was added to precipitate a yellow powder of 3. Yield 0.11 g (95%). Single crystals grew from CH2Cl2-Et2O (3:1) mixtures. 1H NMR (CDCl3, 25 °C): δ = 7.79–7.57 (m, 10H, C6H5), 3.50 (br, 4H, P-CH2-N), 1.41 (s, 3H, C(CH3)3), NH not assigned; 31P-{1H} NMR (CDCl3, 25 °C): δ = 113.8 (s); IR (CH2Cl2): ν(CO) = 2070(s), 2032(vs), 2004(s), 1963(s) cm−1.
References
- Hogarth, G. An unexpected leading role for [Fe2(CO)6(μ-pdt)] in our understanding of [FeFe]-H2ases and the search for clean hydrogen production. Coord. Chem. Rev. 2023, 490, 215174. [Google Scholar] [CrossRef]
- Kleinhaus, J.T.; Wittkamp, F.; Yadav, S.; Siegmund, D.; Apfel, U.-P. [FeFe]-Hydrogenases: Maturation and reactivity of enzymatic systems and overview of biomimetic models. Chem. Soc. Rev. 2021, 50, 1668–1784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rauchfuss, T.B. Synthesis of di-iron(I) dithiolato carbonyl complexes. Chem. Rev. 2016, 116, 7043–7077. [Google Scholar] [CrossRef] [PubMed]
- Apfel, U.-P.; Pétillon, F.Y.; Schollhammer, P.; Talarmin, J.; Weigand, W. Chapter 4 [FeFe] Hydrogenase Models an Overview. In Bioinspired Catalysis; Weigand, W., Schollhammer, P., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 79–103. ISBN 978-3-527-33308-0. [Google Scholar]
- Elleouet, C.; Pétillon, F.Y.; Schollhammer, P. Chapter 17 [FeFe]-Hydrogenases Models. In Advances in Bioorganometallic Chemistry; Hirao, T., Moriuchi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–364. [Google Scholar] [CrossRef]
- Agarwal, T.; Kaur-Ghumaan, S. HER catalyzed by iron complexes without a Fe2S2 core: A review. Coord. Chem. Rev. 2019, 397, 188–219. [Google Scholar] [CrossRef]
- Cheah, M.H.; Borg, S.J.; Bondin, M.I.; Best, S.P. Electrocatalytic proton reduction by phosphido-bridged diiron carbonyl compounds: Distant relations to the H-cluster? Inorg. Chem. 2004, 43, 5635–5644. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.H.; Best, S.P. XAFS and DFT characterisation of protonated reduced Fe-hydrogenase analogues and their implications for electrocatalytic proton reduction. Eur. J. Inorg. Chem. 2011, 2011, 1128–1137. [Google Scholar] [CrossRef]
- Gimbert-Suriñach, C.; Bhadbhade, M.; Colbran, S.B. Bridgehead hydrogen atoms are important: Unusual electrochemistry and poton reduction at iron dimers with ferrocenyl-substituted phosphido bridges. Organometallics 2012, 31, 3480–3491. [Google Scholar] [CrossRef]
- Rahaman, A.; Gimbert-Suriñach, C.; Ficks, A.; Ball, G.E.; Bhadbhade, M.; Haukka, M.; Higham, L.; Nordlander, E.; Colbran, S.B. Bridgehead isomer effects in bis(phosphido)-bridged diiron hexacarbonyl proton reduction electrocatalysts. Dalton Trans. 2017, 46, 3207–3222. [Google Scholar] [CrossRef]
- Das, P.; Capon, J.-F.; Gloaguen, F.; Pétillon, F.Y.; Schollhammer, P.; Talarmin, J.; Muir, K.W. Di-iron aza diphosphido complexes: Mimics for the active site of Fe-only hydrogenase, and effects of changing the coordinating atoms of the bridging ligand in [Fe2{µ-(ECH2)2NR}(CO)6]. Inorg. Chem. 2004, 43, 8203–8205. [Google Scholar] [CrossRef]
- Cheah, M.H.; Borg, S.J.; Best, S.P. Steps along the path to dihydrogen activation at [FeFe] hydrogenase structural models: Dependence of the core geometry on electrocatalytic proton reduction. Inorg. Chem. 2007, 46, 1741–1750. [Google Scholar] [CrossRef]
- Zaffaroni, R.; Rauchfuss, T.B.; Fuller, A.; De Gioia, L.; Zampella, G. Contrasting protonation behavior of diphosphido vs dithiolato diiron(I) carbonyl complexes. Organometallics 2013, 32, 232–238. [Google Scholar] [CrossRef]
- Arrigoni, F.; Rizza, F.; Vertemara, J.; Breglia, R.; Greco, C.; Bertini, L.; Zampella, G.; De Gioia, L. Rational design of Fe2(μ-PR2)2(L)6 coordination compounds featuring tailored potential inversion. ChemPhysChem 2020, 21, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Selan, O.T.E.; Cheah, M.H.; Abrahams, B.F.; Gable, R.W.; Best, S.P. Impact of the 2Fe2P core geometry on the reduction chemistry of phosphido-bridged diiron hexacarbonyl compounds. Austr. J. Chem. 2022, 75, 649–659. [Google Scholar] [CrossRef]
- Shimamura, T.; Maeno, Y.; Kubo, K.; Kume, S.; Greco, C.; Mizuta, T. Protonation and electrochemical properties of a bisphosphide diiron hexacarbonyl complex bearing amino groups on the phosphide bridge. Dalton Trans. 2019, 48, 16595–16603. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Kubo, K.; Kume, S.; Mizuta, T. Formation of a hexacarbonyl diiron complex having a naphthalene-1,8-bis(phenylphosphido) bridge and the Electrochemical Behavior of Its Derivatives. Organometallics 2013, 32, 7011–7024. [Google Scholar] [CrossRef]
- Shi, Y.S.; Yang, W.; Shi, Y.; Cheng, D.-C. Syntheses, crystal structures, and electrochemical studies of Fe2(CO)6(μ-PPh2)(μ-L) (L = OH, OPPh2, PPh2). J. Coord. Chem. 2014, 67, 2330–2343. [Google Scholar] [CrossRef]
- Mai, Y.; Balzen, A.K.; Torres, R.K.; Callahan, M.P.; Colson, A.C. A modular strategy for expanding electron-sink capacity in noncanonical cluster assemblies. Inorg. Chem. 2021, 60, 17733–17743. [Google Scholar] [CrossRef]
- Hayter, R.G. Phosphorus- and arsenic—Bridged complexes of metal carbonyls. VI. Reactions of tetrasubstituted biphosphines and a biarsine with monomeric metal carbonyls. Inorg. Chem. 1964, 3, 711–717. [Google Scholar] [CrossRef]
- Treichel, P.M.; Douglas, W.M.; Dean, W.K. Deprotonation and subsequent alkylation of phosphine-metal carbonyl complexes. Inorg. Chem. 1972, 7, 1615–1618. [Google Scholar] [CrossRef]
- Clegg, W. Crystal structure of bis(µ-bis(trifluoromethyl)phosphido)-hexacarbonyldiiron, Fe2(CO)6[µ-P(CF3)2]2. Inorg. Chem. 1976, 15, 1609–1613. [Google Scholar] [CrossRef]
- Ginsburg, R.E.; Rothrock, R.K.; Finke, R.G.; Coliman, J.P.; Dahl, L.F. The (metal-metal)-nonbonding [Fe2(CO)6(µ2-PPh)2]2− dianion. Synthesis, structural analysis of its unusual dimeric geometry, and stereochemical-bonding implications. J. Am. Chem. Soc. 1979, 101, 6550–6552. [Google Scholar] [CrossRef]
- Collman, J.P.; Rothrock, R.K.; Finke, R.G.; Moore, E.J.; Rose-Munch, F. Role of the metal-metal bond in transition-metal clusters. Phosphido-bridged diiron carbonyl complexes. Inorg. Chem. 1982, 21, 146–156. [Google Scholar] [CrossRef]
- Yu, Y.-F.; Gallucci, J.; Wojcicki, A. Novel mode of reduction of phosphido-bridged, metal-metal-bonded binuclear complexes. Synthesis and reactivity of an unsymmetrical anion from Fe2(CO)6(µ-PPh2)2. J. Am. Chem. Soc. 1983, 105, 4826–4828. [Google Scholar] [CrossRef]
- Walther, B.; Hartung, H.; Reinhold, J.; Jones, P.G.; Mealli, C.; Böttcher, H.-C.; Baumelster, U.; Krug, A.; Möckel, A. Structure and bonding of the coordinatively unsaturated complexes [Fe2(CO)5(µ-PR2)(µ-PR’2)](Fe=Fe) (R = R’ = But; R = Ph, R’ = But). Reaction of Na[Fe2(μ-CO)(CO)6(µ-PR2)] with R’2PCl (R, R’ = Ph, Cy, Me, But). Organometallics 1992, 11, 1542–1549. [Google Scholar] [CrossRef]
- Walther, B.; Hartung, H.; Bambirra, S.; Krug, A.; Böttcher, H.-C. Coordinatively unsaturated complexes [Fe2(CO)5(μ-PBut2)(μ-PR2)](Fe=Fe) (R = Cy, Ph): Addition of PBun3, Ph2PH, and dppm (dppm = bis(diphenylphosphino)methane). The unprecedented complex [Fe2(CO)3(µ-PBut2)(µ-PCy2)(µ-dppm)](Fe—Fe). Organometallics 1994, 13, 172–179. [Google Scholar] [CrossRef]
- Van der Linden, J.G.M.; Heck, J.; Walther, B.; Böttcher, H.-C. Electrochemical investigation of the electron-poor/precise (n = 5/6) complexes [Fe2(CO)n(µ-PR2)(µ-PR’2)] (n = 5, R = R’ = But; n = 6, R = R’ = Ph; R= But, R’ = Ph; R= But, R’ = Cy). EPR study of the radical anion [Fe2(µ-PBut2)2(CO)5]−. Inorg. Chim. Acta 1994, 217, 29–32. [Google Scholar] [CrossRef]
- Flood, T.C.; DiSanti, F.J.; Campbell, K.D. Stereochemical Lability of (CO)3Fe(µ-ER2)2Fe(CO)2PR3. Evidence for two distinct CO averaging arocesses without bridge opening. Inorg. Chem. 1978, 17, 1643–1646. [Google Scholar] [CrossRef]
- McKennis, J.S.; Kyba, E.P. Linked bis(µ-phosphido) and related ligands for metallic clusters. 1. Application to the hexacarbonyldiiron moiety. Organometallics 1983, 2, 1249–1251. [Google Scholar] [CrossRef]
- Seyferth, D.; Wood, T.G.; Fackler, J.P., Jr.; Mazany, A.M. Intramolecular nucleophilic attack at iron in an anionic phosphido-bridged Fe2(CO)6 complex. Organometallics 1984, 3, 1121–1123. [Google Scholar] [CrossRef]
- Rheingold, A.L. A linked diphosphido iron carbonyl complex, μ-P,P’-diphenyltrimethylenebis(phosphido)-μ-P:μ-P’-bis(tricarbonyliron)(Fe–Fe), [Fe2(C15H16P2)(CO)6]. Acta Crystallogr. 1985, C41, 1043–1045. [Google Scholar] [CrossRef]
- King, R.B.; Wu, F.-J.; Sadani, N.D.; Holt, E.M. (Carbonylbis((dialkylamino)phosphido))hexacarbonyldiiron complexes: Migration of a carbonyl group from iron to phosphorus. Inorg. Chem. 1985, 24, 4449–4450. [Google Scholar] [CrossRef]
- King, R.B.; Wu, F.-J.; Holt, E.M. Novel ((diisopropylamino)triphosphine)hexacarbonyldiiron complexes. Inorg. Chem. 1986, 25, 1733–1734. [Google Scholar] [CrossRef]
- Kyba, E.P.; Davis, R.E.; Clubb, C.N.; Liu, S.-T.; Aldaz Palacio, H.O.; McKennis, J.S. Linked bis(µ-phosphido) and related ligands for metallic clusters. 5. Evidence for a highly selective backside attack by strong nucleophiles on a μ-phosphido center. Organometallics 1986, 5, 869–877. [Google Scholar] [CrossRef]
- Kyba, E.P.; Kerby, M.C.; Rines, S.P. A Convenient synthesis of symmetrical and unsymmetrical 1,2-bis(phosphino)benzenes as ligands for transition metals. Organometallics 1986, 5, 1189–1194. [Google Scholar] [CrossRef]
- De, R.L.; Wolters, D.; Vahrenkamp, H. Darstellung und reaktionen des tetrahedran-moleküls Fe2(CO)6(P-tert-C4H9)2/Preparation and reactions of the tetrahedrane molecule Fe2(CO)6(P-tert-C4H9)2. Z. Naturforsch. B 1986, 41, 283–291. [Google Scholar] [CrossRef]
- Seyferth, D.; Wood, T.G. Michael-type addition reactions of bis(µ-phenylphosphido)bis(tricarbonyliron) with olefinic α,β-unsaturated carbonyl compounds. Construction of chelating bis(phosphido) ligands. Organometallics 1987, 6, 2563–2567. [Google Scholar] [CrossRef]
- King, R.B.; Wu, F.-J.; Holt, E.M. Dialkylamino phosphorus metal carbonyls. 4. Novel phosphorus-bridging carbonyl derivatives and triphosphine derivatives from reactions of tetracarbonylferrate(-II) with (dialkylamino)dichlorophosphines. J. Am. Chem. Soc. 1987, 109, 7764–7775. [Google Scholar] [CrossRef]
- King, R.B.; Wu, F.-J.; Holt, E.M. Dialkylamino phosphorus metal carbonyls. 5. Chemical reactivity of the phosphorus-bridging carbonyl group in carbonylbis[(diisopropylamino)phosphido]hexacarbonyldi-iron. J. Am. Chem. Soc. 1988, 110, 2775–2782. [Google Scholar] [CrossRef]
- Seyferth, D.; Wood, T.G.; Henderson, R.S. Reactions of lithium bis(µ-phenylphosphido)-bis(tricarbonyliron),(µ-PhPLi)2Fe2(CO)6, with organic halides. A novel anionic rearrangement of a chelating diphosphido ligand. J. Organomet. Chem. 1987, 336, 163–182. [Google Scholar] [CrossRef]
- Seyferth, D.; Wood, T.G. Michael-type addition reactions of bis(phenylphosphido)bis(tricarbonyliron) with acetylenic α,β-unsaturated carbonyl compounds: Multiple reaction pathways. Organometallics 1988, 7, 714–718. [Google Scholar] [CrossRef]
- Kumar, V.; Newton, M.G.; King, R.B. Insertion of a carbenoid unit into an Fe2P2 cluster. J. Organomet. Chem. 1994, 472, C13–C14. [Google Scholar] [CrossRef]
- Baik, M.-H.; Ziegler, T.; Schauer, C.K. Density functional theory study of redox pairs. 1. Dinuclear iron complexes that undergo multielectron redox reactions accompanied by a reversible structural change. J. Am. Chem. Soc. 2000, 122, 9143–9154. [Google Scholar] [CrossRef]
- Burdett, J.K. A molecular-orbital study of some di-phosphido-bis(tricarbony1iron) complexes. The importance of metal-bridging ligand interactions in determining molecular geometry. J. Chem. Soc. Dalton Trans. 1977, 5, 423–428. [Google Scholar] [CrossRef]
- Garrou, P.E. ΔR-ring contributions to phosphorus-31NMR parameters of transition-metal-phosphorus chelate complexes. Chem. Rev. 1981, 81, 229–266. [Google Scholar] [CrossRef]
- Bartsch, R.; Hietkamp, S.; Morton, S.; Stelzer, O. Reaktionen koordinierter liganden: X. Reaktivität zweikerniger eisencarbonylkomplexe mit sekundären phosphidobrüken μ-rph. J. Organomet. Chem. 1981, 222, 263–273. [Google Scholar] [CrossRef]
- Lawrence, J.D.; Li, H.; Rauchfuss, T.B. Beyond Fe-only hydrogenases: N-functionalized 2-aza-1,3-dithiolates Fe2[(SCH2)2NR](CO)x (x = 5, 6). Chem. Commun. 2001, 16, 1482–1483. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).